World's thinnest gold leaf, dubbed 'goldene,' is just 1 atom thick

Scientists have created the world's thinnest gold leaf, which is just a single atom thick.
The new material, dubbed "goldene," could have important applications in carbon dioxide conversion and hydrogen generation, the researchers said.
To make goldene, the team employed a 100-year-old technique used by Japanese iron smiths to isolate single layers of the precious metal. They reported their work in the journal Nature Synthesis on April 16.
Researchers are particularly interested in two-dimensional materials because of their unusual optical, electronic and catalytic properties. The extremely high surface area of these substances relative to their volume means they behave very differently than chemically-identical bulk solids, and numerous examples of 2D materials have been reported since the discovery of graphene in 2004.
Related: World's thinnest electronic device is 2 atoms thick
However, most of these materials are prepared from nonmetals or mixed compounds, and creating single-atom sheets of pure metals is much more challenging.
"Metals do not like to be lonely," Michael Yeung, a solid-state chemist at the University at Albany, told Live Science in an email. "Because the bonding in metals is delocalized, they readily will bond into themselves and agglomerate. Preparing a single layer is quite a feat because you are fighting against the metal's desire to bond with not only itself but with other sheets."
Previous attempts have run into this problem. Several teams have created a single layer of gold atoms embedded within a supporting solid, such as graphene-coated silicon carbide — "like a sort of 'sandwich' structure, using graphene as a pseudo-bread and the gold as the meat," Yeung said. But extracting the goldene from these complex layered solids proved problematic, with the gold atoms coagulating into nanoparticles as soon as the support was removed.
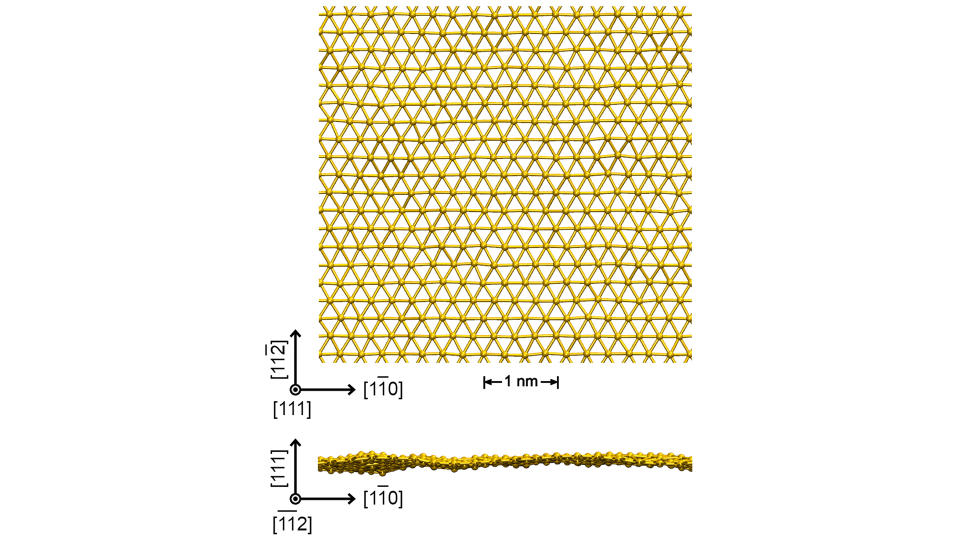
Shun Kashiwaya, an assistant professor in the Department of Physics, Chemistry and Biology at Linkӧping University in Sweden, and colleagues turned this approach on its head to successfully isolate goldene sheets for the first time.
They began by creating a layered structure of titanium, silicon and carbon, which they then covered with a surface layer of gold. Over 12 hours, gold particles diffused into the material, replacing the silicon layer with gold and creating a goldene sheet embedded within the solid. However, rather than trying to remove the gold layer, the team carefully etched away all of the surrounding solid, leaving the gold sheet untouched.
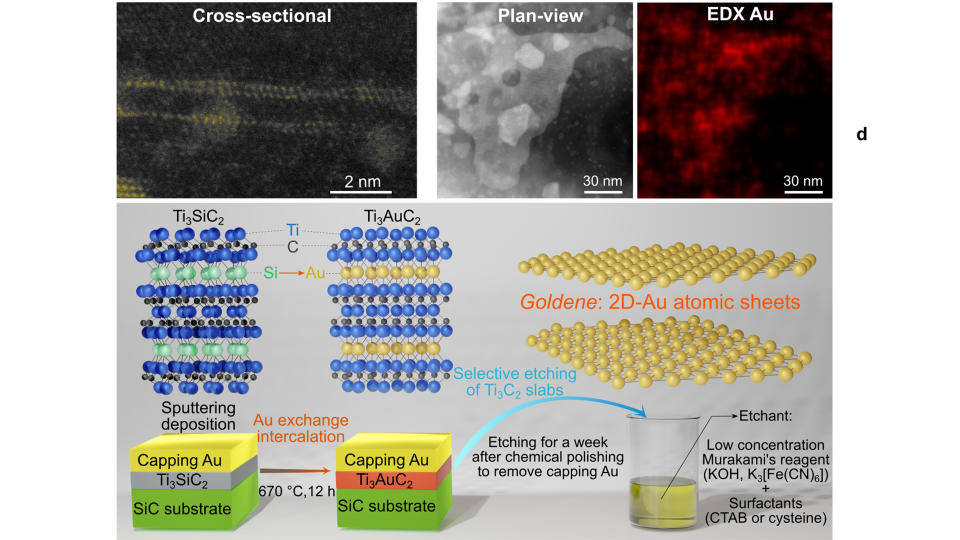
They figured out the technique when study coauthor Lars Hultman, a professor in the Department of Physics, Chemistry and Biology at Linkӧping University, was researching chemical etchants. Hultman found a 100-year-old method used by Japanese smiths to etch away carbide residues in steel, Kashiwaya told Live Science. Called Murakami's reagent or alkaline potassium ferricyanide, the solution etched away the surrounding titanium carbide support, without affecting the goldene sheet.
To perfect the method, the team experimented with different reaction conditions and concentrations of the etching solution. Crucially, they found that adding a cysteine as a surfactant, or a chemical which decreases the surface tension of a liquid, stabilized the isolated sheets and prevented the gold atoms from clustering and combining into nanoparticles.
The freestanding goldene sheets were up to 100 nanometers long and are hundreds of times thinner than ordinary gold leaf.
RELATED STORIES
—Bizarre 'nanoseaweed' is the thinnest gold in the world
Kashiwaya and Hultman believe that, due to goldene's enhanced chemical reactivity, it could have important applications in reactions to convert carbon dioxide into fuels such as ethanol and methane and water into hydrogen. They are currently working on improving the synthetic method.
"We aim to explore goldene's fundamental physical and chemical properties and further develop the synthetic process to increase both the goldene sheet area and yield," Kashiwaya said. "We also envision applying this approach to produce other elemental 2D materials (metallenes) beyond goldene."
Yeung is particularly interested in the preparation of new 2D materials made possible by this method. "The ability to selectively etch what is normally stable means that a bunch of new materials can be made," he said.
The next step could be creating a single layer of silver using aluminas as the base, Yeung said.

