'Frankenstein' mice with brain cells from rats raised in the lab
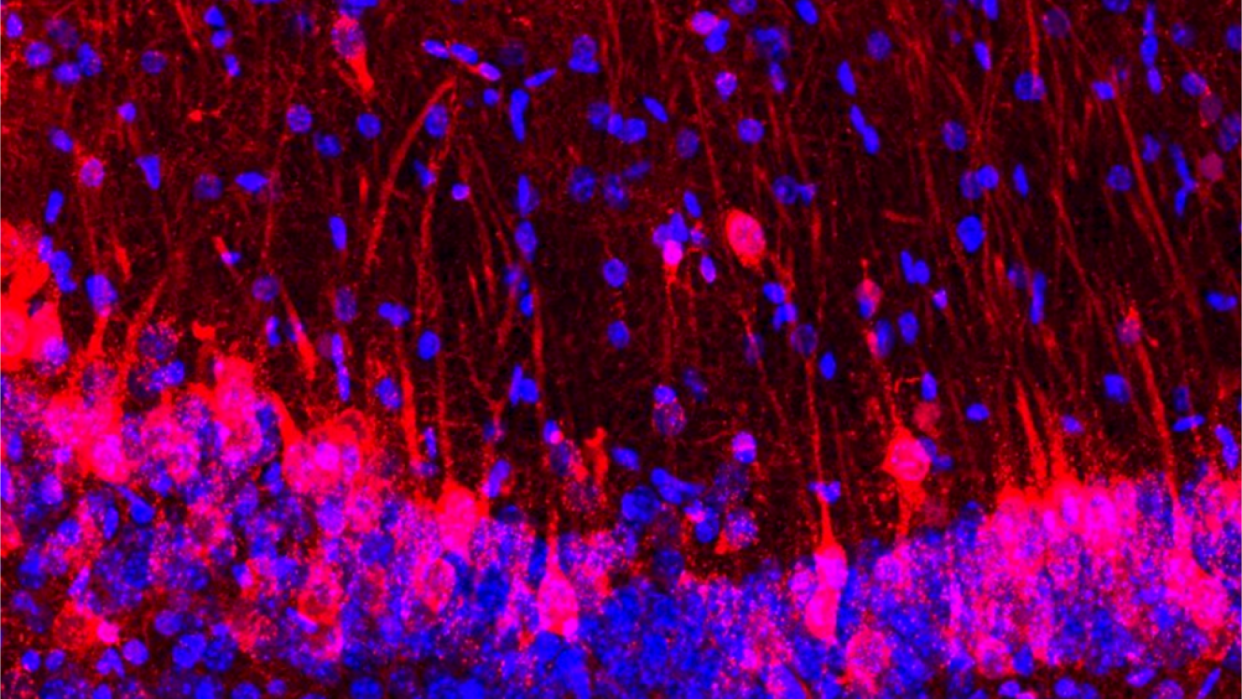
In an experiment reminiscent of "Frankenstein," scientists found that rat brain cells can fill in for lost neurons in mice, even allowing the host rodents to sniff out sweets.
While splicing rat and mouse brains together may sound odd, this work aims to build a basis for understanding how mammal brains develop, said Kristin Baldwin, a neuroscientist at Columbia University and the lead author of a new study describing the experiment.
Baldwin and her team's study, which was published in the journal Cell alongside a second study from collaborators at the University of Texas (UT) Southwestern, shows that the rat brain cells introduced into a mouse brain pick up cues from their new environment. These cells develop in the same time frame as nearby mouse brain cells, communicating with them and even adjusting their size to match.
"The host is controlling at least two aspects: the size and also the developmental speed," said Jun Wu, a molecular biologist at the UT Southwestern Medical Center and the lead author of the second study. "That's very interesting and suggests the microenvironment has influence on the pace, as well as the size, of the donor cell."
Related: Rat brain injuries 'plugged' with lab-grown human minibrains in world-first experiment
The study led by Baldwin focuses on how networks form in a hybrid mouse-rat brain, while the study led by Wu focuses more on replacing an entire brain region with transplanted cells. The research could lead to other cross-species brain tissue, helping scientists study brain development and disease and potentially develop new treatments for people.
Baldwin's team first used bacterial toxins to either kill or silence brain cells in developing mouse embryos. They started when the developing embryo was just a hollow ball of 100 to 200 cells, called a blastocyst, and targeted cells involved in sensing scents. Into these blastocysts, they also injected stem cells from rats, using a type of cell capable of developing into many cell types.
They then implanted the altered blastocysts into mouse mothers and allowed the embryos to develop. They found that the rat cells developed apace with the mouse cells, filling in for the killed or silenced cells in the scent-sensing centers of the brain. Completely wiping out the mouse cells and replacing them with rat cells led to some odd-looking anatomy, Baldwin told Live Science, but the mouse's sense of smell still worked completely normally.
The fact that the different neurons foraged a network together and gave rise to fairly normal behavior is promising, Baldwin said. There are hopes right now for treating brain diseases, such as Parkinson's or Alzheimer's, with donated or lab-grown cells that would replace the diseased cells in patients' brains.
Similar donations of brain tissue are a long way off — but there's a need to ensure that neuron transplants of that sort could actually lead to functional brain networks.
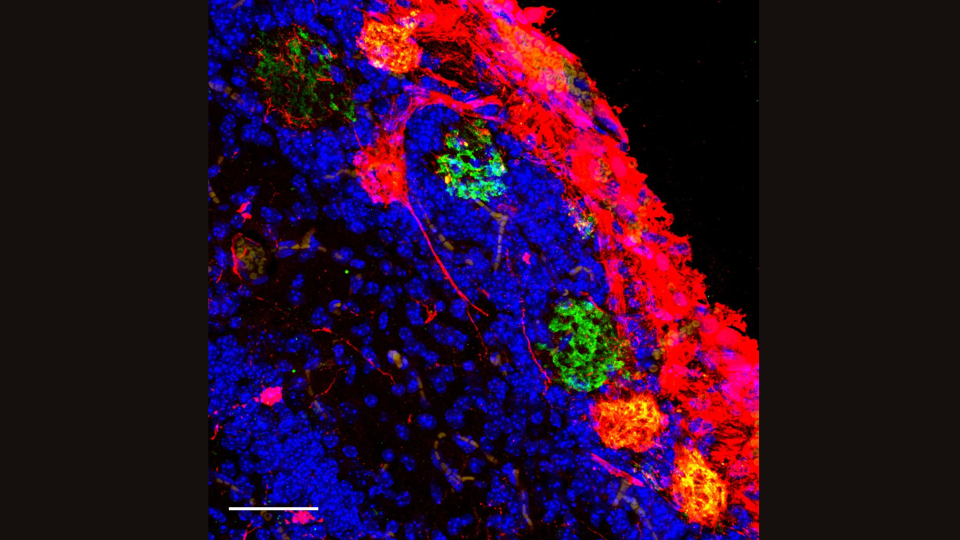
"You could say, 'We can replace the cells that make dopamine and they will make dopamine,'" Baldwin told Live Science. Dopamine is a chemical messenger that's dramatically depleted in Parkinson's. "But what are they doing to the information processing in that part of the brain?" Baldwin added. "Are they participating in the right way, and could we improve that?"
Related: Will brain transplants ever be possible?
Wu's study focused on replacing an entire region of the mouse brain with rat cells. The team used the gene-editing technique CRISPR to shut down a gene that triggers the development of the mouse forebrain in the womb. They replaced this large brain region with rat cells, and 60% of the cells in the mature mice ended up being of rat origin. Despite their hybridized brains, the mice acted like typical lab mice.
RELATED STORIES
—Kaleidoscopic image of a mouse's brain is 64 million times sharper than a typical MRI
—Neurons aren't the only cells that make memories in the brain, rodent study reveals
—In a 1st, scientists combine AI with a 'minibrain' to make hybrid computer
"We show that up to 60% of cells that are coming from a different species in the forebrain doesn't really dramatically alter the behavior of the host recipient," Wu told Live Science.
No one's planning to put human neurons in mouse brains. That would raise far more ethical issues than growing hybrid rodent-rodent brains, because the brains could cross a threshold and become "too human." In any case, it would be far more technically difficult to achieve, Wu said. There have been attempts to grow other human organs in animals — for instance, scientists grew human kidneys inside pig embryos — but brain tissue would be another matter.
Researchers could theoretically apply these techniques to hybridize the brains of different monkey species. This could make it easier to genetically tweak the primates to model aspects of human diseases; that's because different gene-modification techniques tend to be tried and tested in specific species and aren't always easy to use across species.
Such work in monkeys might be more relevant to people, as many diseases that humans get don't affect mice or rats, Baldwin noted. But it would raise its own ethical questions.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

