CDC investigating fake Botox, blamed for 9-state outbreak of botulism
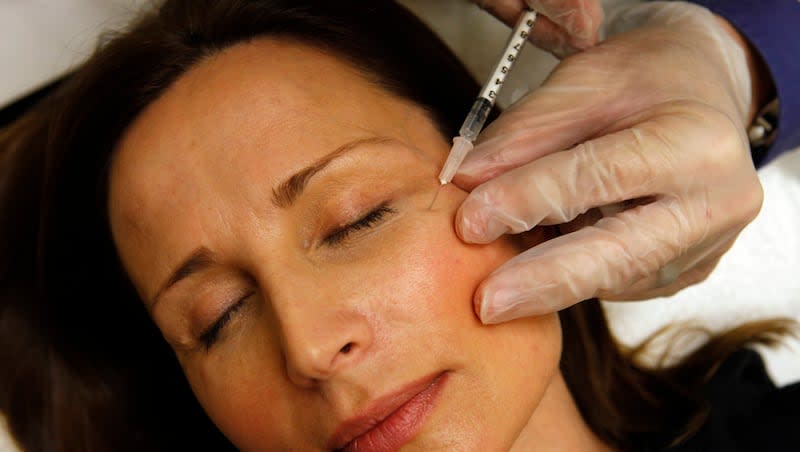
Counterfeit or mishandled Botox has been linked to 19 bad reactions that in some cases have hospitalized people with botulism. Now national public health organizations are warning people to be sure they know the source of any Botox injection and only get the cosmetic treatment from licensed individuals.
Botox injections contain botulinum toxin and are sometimes used to paralyze muscles for a period of time. Cosmetically, it’s most often used to reduce wrinkles. The Mayo Clinic said they are also used for neck spasms, sweating, overactive bladder, lazy eye and other conditions and might help prevent migraine headaches. It, too, cautions people to select the person who will provide a Botox injection carefully.
With 19 people affected in at least nine states — nine of those individuals hospitalized — officials at the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration are emphasizing the need to avoid injections from unlicensed or untrained individuals or those given in non-health-care settings, including homes and spas.
Which states have reports about fake Botox?
Cases linked to bogus Botox have been reported in Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee and Washington. Investigations are ongoing into the source of the products.
Four of the nine received botulism antitoxin after they tested positive for botulism. The other five tested negative. Per the CDC, botulism is a rare but serious illness from Clostridium botulinum that attacks nerves and makes breathing difficult, paralyzes muscles and can kill. While the bacteria can be naturally occurring and generally harmless, in certain conditions it can become extremely dangerous. One of the most common sources of botulism is food that has been improperly home canned, preserved or fermented.
Those affected have reported symptoms including blurred or double vision, drooping eyelids, difficulty swallowing, dry mouth, slurred speech, difficulty breathing, fatigue and general weakness, per the CDC and FDA, which notes some people have trouble lifting their head after receiving an injection of bogus Botox.
The FDA said anyone experiencing any of those symptoms after receiving an injection of botulinum toxin products should call their doctor or go to an emergency room.
“These incidents have occurred when counterfeit Botox is injected by licensed and unlicensed individuals and/or in non-medical or unlicensed settings,” per the FDA advisory. “The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe,” the notice read.
Spotting the counterfeit Botox
Both agencies offer tips to reduce the risk of getting a fake injection that could be harmful, including:
Ask if the product has received FDA approval and whether the product’s source is reliable.
If your state has a tool that lets consumers look up licensing, do so.
The FDA noted several similarities between the counterfeit products and FDA-approved Botox, which is made by AbbVie in 50-, 100- and 200-unit forms. The agency said that there’s no indication that the reported adverse events are related to AbbVie’s FDA-approved Botox, which “should be considered safe and effective for its intended and approved uses.”
The agency further noted that the counterfeit product is packaged in a counterfeit carton and vial and might contain these markings:
The outer carton and vial are labeled C3709C3.
The active ingredient is “Botulinum Toxin Type A,” instead of “OnabotulinumtoxinA.”
The marked unit indicates 150-unit doses, which are not something AbbVie or Allergan (an AbbVie company) make.
The outer carton is not solely in English.
Consumers can see photos of the bogus Botox online on the FDA counterfeit announcement page, which also contains information about reporting adverse reactions to any medication.
This is not the first time that faux Botox has caused problems. Back in 2005, a company was charged with crimes after as many as 1,000 people received the counterfeit injections, as The Associated Press reported. At that time, health experts were saying people should avoid Botox “parties.” In 2006, a husband-and-wife doctor team was sent to prison for selling dangerous bootleg Botox.

