Why the FDA said no to needle-free EpiPen injection alternative
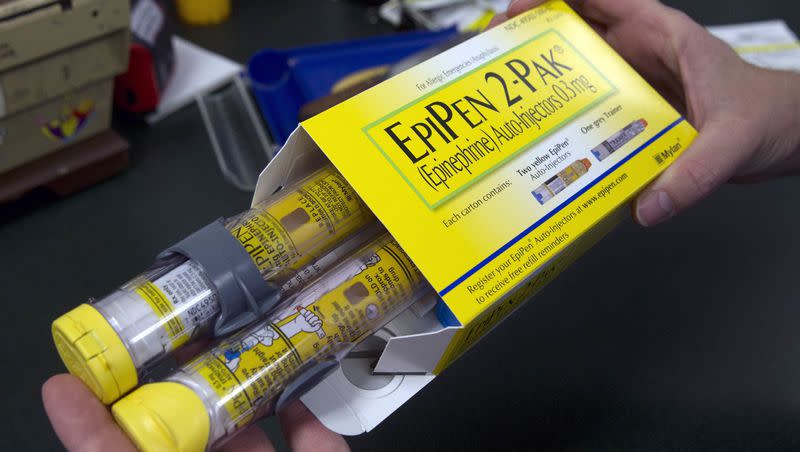
Those hoping for an alternative to the EpiPen’s needle bite when they’ve been exposed to bees or other serious allergens will have to hold on — at least for now. The U.S. Food and Drug Administration withheld approval pending more study of the first nasal spray that would have been a needle-free alternative to injectable epinephrine.
NBC News called it a “surprise move.”
In May, it appeared that the nasal spray, called neffy, was cruising toward approval, since the FDA advisory committee did not recommend additional trials, according to ARS Pharma, which developed the medication.
But a release from the company says that the “FDA now requests a repeat-dose study be completed prior to neffy approval as opposed to a previously agreed-upon post-marketing requirement.”
Right now, people can only use epinephrine autoinjectors, including EpiPen, if they are having a severe allergic reaction. The manufacturer of neffy believes that people would be more apt to take along a nasal spray and would find it more convenient. And some people are afraid of needles.
Committee recommended neffy
Epinephrine became a common remedy for severe allergic reactions, including anaphylaxis, from exposure to common allergens like bees, peanuts and pet dander. The reaction can be lethal.
Related
“I’m shocked,” Dr. Zachary Ruben, an allergist at Oak Brook Allergists in Illinois, told NBC regarding the decision.
ARS Pharma said it plans to submit “a formula dispute resolution request” to appeal the decision laid out by the regulatory agency in a letter.
WWLP television noted that it is “rare” for the FDA to not approve drugs recommended by its advisers.
In a news release, ARS Pharma reported that in May the advisory committee voted 16-6 to recommend neffy be approved for adults and 17-5 that it be approved for children weighing 30 kilograms (66 pounds) or more. None of them raised concerns that indicated additional testing, the release said.
ARS Pharma pushes back
“We are very surprised by this action and the late requirement at this time to change the repeat-dose study from a post-marketing requirement, which we had previously aligned on with FDA, to a pre-approval requirement, particularly given the positive advisory committee vote,” said Richard Lowenthal, co-founder, president and CEO of ARS Pharma. “In fact, multiple committee members highlighted the favorable profile of neffy in our completed single-dose nasal allergy challenge study and that any decline in exposure 20 minutes after dosing, after the unexpected response period, is of no concern.”
One problem was the drug could not be studied in a randomized controlled trial of people who were experiencing anaphylaxis. It would not be ethical to randomize people into different treatment modes in that situation.
Dr. Maryann Amirshahi, a professor of emergency medicine at Georgetown University School of Medicine and a member of the advisory committee, told NBC the lack of data in patients with anaphylaxis was a sticking point. She voted against the drug both times, the article said.
“I listened to the parents in the hearing and I heard them talk about how traumatic it was to give their children a shot,” Amirshahi said in an email to NBC News on Tuesday. “But to me, as a parent, panel member, pharmacologist and ER doc who has seen her fair share of anaphylaxis, the scary thing was not the shot, but a drug failing in treating a life-threatening condition.”
She told NBC it might be possible to study the drug on people experiencing severe allergic reactions in an ER or an allergist’s office, with a proven treatment at the ready in case.

