The Right Way to Brine Turkey
Our guide tells you everything you need to know about brining a turkey, and why dry-brining delivers juicy meat with more intense flavor.
Let me start this off by saying that I don't brine my meat. Ever. Not for Thanksgiving, not for my Sunday supper, and certainly not for a quick weeknight meal. This post is about the reasons why.
Serious Eats / J. Kenji López-Alt
It seems to me that, as recently as 15 years ago, dry turkey was a given. The yearly Thanksgiving ritual at my family's table did not include any ill-mannered offspring crying out, "DAAAAaaaad, Mom ruined the turkey again"—turkey wasn't something that could be ruined. It was dry, tough, and stringy, and that was a fact of life.
Then, about a decade ago, brining entered the scene.* Thanks to an overnight soak in a saltwater solution, gone were the days of dry breast meat and extra servings of gravy. I, for one, welcomed our new moist-breasted overlords. Even my mother could throw a turkey in the oven and pull out something edible a few hours later. It was positively magical!
*Or, more accurately, the technique that had been known to large swaths of the populated world, including China and Scandinavia, for millennia finally made inroads into North America's holiday menu.
These days, everybody and their grandmother (better known as the typical Thanksgiving gathering) has heard of brining, and more and more folks are doing it at home before Turkey Day. But it's not all pie and gravy. There are a few distinct and definite downsides to wet brining, and many folks are making the switch to dry brining (a.k.a. extended salting). The question is, which method works best?
How Brining Works
Before we get too far ahead of ourselves, let's do a quick recap on brining basics. The basic process involves soaking meat (usually lean meats, like turkey, chicken, or pork chops) in a tub full of heavily salted water overnight. Most brines are in the range of 5 to 8% salt to water by weight. Over the course of the night, the meat absorbs some of that water. More importantly, that water stays put even after the meat is cooked. By brining meat, you can decrease the amount of total moisture loss by 30 to 40%.
To demonstrate, I cooked three identical turkey breasts in a 300°F (150°C) oven to an internal temperature of 145°F (63°C). One was brined, the other was soaked overnight in plain water, and the last was left alone. All three breasts came from non-kosher, non-enhanced birds (i.e., the birds were natural, having received no treatment after slaughter). I charted their weight straight from the package, after brining, and after cooking.
Both the bird soaked in brine and the bird soaked in water gained a significant amount of weight prior to roasting, but while the watered bird lost nearly all of that weight as it cooked, the brined bird retained a good deal more. This corresponded to a juicier texture on eating. So what's going on here?

Serious Eats / J. Kenji López-Alt
Some publications attribute it all to osmosis—the tendency for water to move across a membrane from an area of low solute concentration to an area of high solute concentration. In this case, water moves from the brining vessel (low solute concentration) to the inside of the turkey's cells (where there are lots of proteins, minerals, and other fun biological goodies dissolved in the water).
This theory is, in fact, inaccurate. If it were true, then soaking a turkey in pure, unsalted water should be more effective than soaking it in a brine, and we've already seen that that is not the case. Moreover, if you soak a turkey in a ridiculously concentrated brine (I tested turkey in a 35% salt solution), according to the osmosis theory, it should dry out even more.

Serious Eats / J. Kenji López-Alt
However, I found that despite turning the turkey inedibly salty, a highly concentrated 35% salt solution was just as effective at helping a turkey retain moisture as a more moderate 6% salt solution, indicating that the osmosis theory is entirely bunk.
To understand what's really happening, you have to look at the structure of turkey muscles. Muscles are made up of long, bundled fibers, each one housed in a tough protein sheath. As the turkey heats, the proteins that make up this sheath will contract. Just like when you squeeze a tube of toothpaste, this causes juices to be forced out of the bird. Heat them to much above 150°F (66°C) or so, and you end up with dry, stringy meat.
Salt helps mitigate this shrinkage by dissolving some of the muscle proteins (mainly myosin). The muscle fibers loosen up, allowing them to absorb more moisture, and, more importantly, they don't contract as much when they cook, ensuring that more of that moisture stays in place as the turkey cooks.
Sounds great, right? But there's a catch.
The Problems With Brining
There are two major problems with brining. First off, it's a major pain in the butt. Not only does it require that you have a vessel big enough to submerge an entire turkey (common options are a cooler, a big bucket, or a couple of layers of heavy-duty garbage bag, tied together with hopes and prayers against breakage), but it requires that you keep everything inside it—the turkey and the brine—cold for the entire process. For an extra-large bird, this can be a couple of days, meaning that you've either given up using the main compartment of your fridge at the time of year that you most want to use it, or that you keep a constant supply of ice packs or ice rotating to keep that bird cold.
""brining robs your bird of flavor""
Second, brining robs your bird of flavor. Think about it: Your turkey is absorbing water, and holding on to it. That means that that extra 30 to 40% savings in moisture loss doesn't really come in the form of turkey juices—it's plain old tap water. Many folks who eat brined birds have that very complaint: It's juicy, but the juice is watery.
I've seen a number of solutions (solutions, get it? haha) offered for this problem, so I decided to test them all out side by side.
Brining Alternatives
By far the most common alternative is plain old salting. When you salt a turkey (or chicken) breast, meat juices are initially drawn out through the process of osmosis (yes, this time it really is osmosis at work). As the salt dissolves in these juices, it forms what amounts to a very concentrated brine, which then allows it to break down muscle proteins. The loosened muscle fibers allow the juices to get reabsorbed, this time taking the salt along for the ride.
Through this process—osmosis, dissolving, reabsorbing—the salt will slowly work its way into the meat.
I've also heard people ask the very obvious question: If brining introduces bland, boring tap water into the bird, why not brine in a more flavorful solution?
Why not, indeed? I decided to find out.
With so many methods to test side by side, it became impractical to try to roast turkey breasts simultaneously. Instead, I roasted 24 chicken breasts in four different batches of six, averaging out the data across the batches. While chicken is not exactly turkey, the two are similar enough that results for one should correlate to results for the other.

Serious Eats / J. Kenji López-Alt
Here's what I tried:
Breast #1: plain (untreated)
Breast #2: brined overnight in a 6% salt solution
Breast #3: heavily salted overnight
Breast #4: brined overnight in chicken broth with a 6% salt content
Breast #5: brined overnight in cider with a 6% salt content
Breast #6: soaked overnight in plain water
Breasts #1 and #6 were included as a control to ensure that the brine and salt solutions were behaving as expected, as well as a means of evaluating how closely the data would mirror that of the turkey breasts.
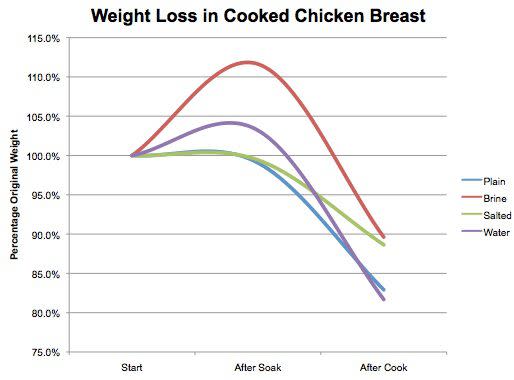
Here's what happened with breasts #1, #2, #3, and #6 (plain, brined, salted, and water-soaked).
Serious Eats / J. Kenji López-Alt
As expected, the brined chicken breasts held on to significantly more moisture than either the plain chicken breasts or the water-soaked chicken breasts. Indeed, in this test, the water-soaked breasts actually ended up drier on average than the plain breasts. Take a look at the carnage:

Serious Eats / J. Kenji López-Alt
Dry as the Gobi Desert (on an admittedly very-moist-for-a-desert day).
On the other hand, take a look at the brined breast:

Serious Eats / J. Kenji López-Alt
As plump and juicy as a benevolent aunt in a Disney film. Tasting it, you definitely feel a case of wet-sponge syndrome. Water comes out of it as you chew, giving you the illusion of juiciness, but the texture is a little too loose, and the flavor a little bland.
Moving on to the salted breast, we find that it's still significantly moister than the unsalted breast (though it was a couple of percentage points drier than the brined breast). Tasting it, you'll find it's undoubtedly juicier and better-seasoned, with a stronger chicken flavor. Texture-wise, it's significantly different from both plain and brined turkey, with the smooth, dense-but-tender texture of lightly cured meat.
Visually, you can see clear signs of this curing with its decidedly pink hue:

Serious Eats / J. Kenji López-Alt
With a small chicken breast, this pink, moist, cured section extends nearly to the center of the breast. On a turkey, you'd see it only around the outer edges (which, serendipitously, happen to be the parts most prone to overcooking and drying out anyway).
While the brined breast was slightly juicier, flavor-wise and texture-wise, I'd take the salted chicken over the brined any day.
What About the Flavored Brines?
First off, don't try to brine your turkey or chicken in cider (or any other acidic marinade, for that matter). Don't do it. Just don't. The acid in the cider will kick off the denaturization process in the meat, effectively "cooking" it without heat. The results? Ultra-dry meat, with a wrinkled, completely desiccated exterior, like this:

Serious Eats / J. Kenji López-Alt
More interesting were the results of the broth-soaked chicken. It seems like the ultimate solution, right? If brining forces bland water into your meat, why not replace that water with flavorful broth?
Unfortunately, physics is a fickle mistress who refuses to be reined in. When I tasted the broth-soaked chicken next to the plain brine-soaked chicken, there was barely a noticeable difference in flavor at all. The broth-soaked chicken still had the same hallmarks of a regular brined bird (juicy/wet texture, blander flavor). What the heck was going on?
There are two principles at work here. The first is that, while broth is a pure liquid to the naked eye, broth actually consists of water with a vast array of dissolved solids in it that contribute to its flavor. Most of these flavorful molecules are organic compounds that are relatively large in size—on a molecular scale, that is—while salt molecules are quite small. So, while salt can easily pass across the semipermeable membranes that make up the cells in animal tissue, larger molecules cannot.**
** Good thing, too; otherwise, you'd be leaking proteins and minerals out of your body every time you took a bath.
Additionally, there's an effect called salting out, which occurs in water-based solutions containing both proteins and salt. Think of a cup of broth as a college dance party populated with cheerleaders (the water—let's call them the Pi Delta Pis), nerds (the proteins—we'll refer to them as the Lambda Lambda Lambdas), and jocks (the salt—obviously the Alpha Betas).***
*** I make no specifications as to the gender and sexual preferences of said classes of individuals, but for the sake of this analogy, let us assume that nerds and jocks are not attracted to each other and that cheerleaders attract both nerds and jocks.
Now, at a completely jock-free party, the nerds actually have a shot at the cheerleaders, and end up commingling with them, forming a homogeneous mix. Open up the gymnasium doors, and a few of those cheerleaders will leave the party, taking a few nerds along for the ride. Unfortunately, those gymnasium doors are locked shut, and the only folks strong enough to open them are the jocks. So what happens when you let some jocks into that party?
The cheerleaders, who were initially fine socializing with the nerds, will quickly and selectively flock to the jocks. The nerds end up finding each other, huddling into small groups, and twiddling their thumbs. When the jocks finally go to bust the gymnasium doors open at the end of the party, they leave hand in hand with the cheerleaders, leaving the nerds in the dust. In our sad tale, those Tri-Lambs never get their revenge.
The exact same thing is happening in a broth-based brine. Water molecules are attracted to salt ions and will selectively interact with them. The poor proteins, meanwhile, are left with only each other, and end up forming large aggregate groups, which makes it even harder for them to get into the meat. When the salt breaks down muscle fibers sufficiently to allow the uptake of water (the equivalent of our jocks breaking down those doors), plenty of water and salt gets into the meat, but very little protein does.****
The result? Unless you're using an extra-concentrated homemade stock, the amount of flavorful compounds that make it inside your chicken or turkey is very, very limited. Given the amount of stock you'd need to use to submerge a turkey, this doesn't seem like a very wise move.
**** This phenomenon is used in biology to remove specific unwanted proteins from solutions. As more salt is added to a solution, proteins will form larger and larger aggregates, until they are eventually large enough to be visible to the naked eye and precipitate out of the solution. Those proteins can then be removed with centrifugation. By knowing the salt concentration that causes different proteins to precipitate, scientists can target specific proteins to be removed, while keeping the rest in solution. The excess salt can then be removed via dialysis (essentially microscopic straining).
So What Does This Mean for My Turkey?
This is all well and good, but what does it mean? How do you apply it?
Well, let me end how I started: I don't brine my birds, because I like my birds to taste like birds, not like watered-down birds. Salting your meat is nearly as effective at preventing moisture loss, and the flavor gains are noticeable. Want to know the truth? Even advance salting is not a necessary first step. I see it more as a safeguard against overcooking. It provides a little buffer in case you accidentally let that bird sit in the oven an extra 15 minutes. As long as you are very careful about monitoring your bird, there's no reason to brine or salt it in advance.
That said, it doesn't hurt to take precautions.
How to Do It if You So Choose
We've put together a handy quick and dirty guide to brining and dry brining, which lays it all out in detail.
Let deliciousness, merriment, and family bonding ensue. You may not all be able to agree on whether the cranberries belong in the stuffing or on the side, but at least you can all agree that this is one darn tasty bird.
Editor's Note
This story was originally published as part of the column "The Food Lab."
November 2012
Read the original article on Serious Eats.

