Tydemy Birth Control Pills Recalled for Potential 'Reduced Effectiveness'
Lupin / Food and Drug Administration
Fact checked by Nick Blackmer
Two lots of Tydemy birth control pills have been recalled because they may have ”reduced effectiveness.”
The potential ineffectiveness is suspected due to low levels of ascorbic acid in both lots and an additional “known impurity” in one of the lots.
Experts recommend anyone with impacted medication switch to a different, equivalent birth control as soon as possible.
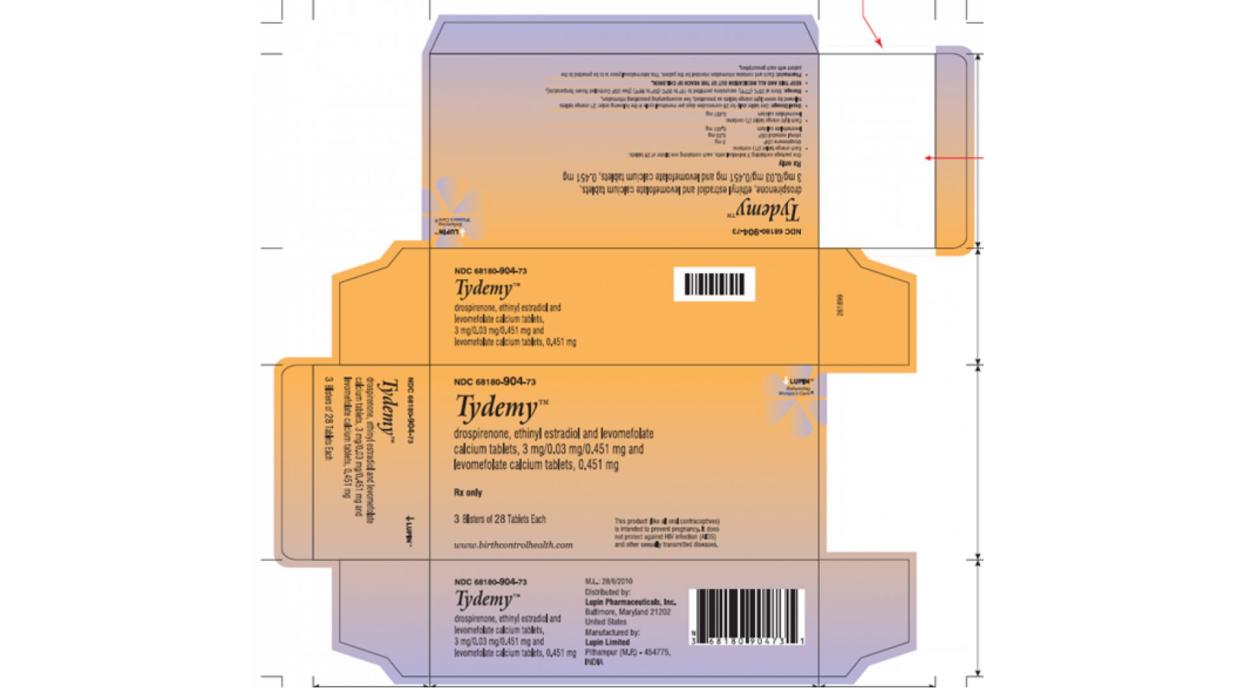
Two lots of Tydemy, the birth control medication, have been recalled because they may not be effective, the Food and Drug Administration announced on Tuesday.
The two recalled lots were distributed nationwide between June 3, 2022 and May 31, 2023, and have the following codes:
L200183, expiry date January 2024
L201560, expiry date September 2024
Lupin, the pharmaceutical company that manufactures Tydemy, said that the recalled pills were sold in the U.S. to mail-order pharmacies, wholesalers, drug chains, and supermarkets. The company alerted its consumers about the voluntary recall on July 29.
The birth control pills were found to contain low levels of one ingredient—ascorbic acid. These low levels could impact the medication’s effectiveness, possibly resulting in unexpected pregnancy for consumers.
Specifically, lot L200183 tested low for ascorbic acid and “high for a known impurity,” Lupin said.
So far, the FDA says it has not received any reports of adverse events from people taking the recalled Tydemy.
Lupin urged wholesalers, retailers, and distributors to stop the sale and distribution of the affected drugs immediately.
Consumers who may have taken the recalled Tydemy should continue to take their medication, Lupin said, but should get in touch with their healthcare provider as soon as possible to find an alternative medication.
Tydemy comes in blister packs of 28 pills, and the recalled lots were sold in sets of one or three-blister packages. The lot numbers can be found on the side of the carton, the company said.
“Find the birth control package that you have at home, or if you don’t have it, you can call your pharmacy and they should be able to let you know if you were given one of the lot numbers that were even affected,” Chelsea Thibodeau, DO, assistant professor of family medicine and community health at the University of Minnesota Medical School, told Health.
Issuing the recall was the safest thing to do, Thibodeau said, but it is out of an abundance of caution.
“Don’t panic,” she advised.
People should also get in touch with their doctor if they think that they may have experienced any health issues due to taking the recalled birth control pills—these can be reported to the FDA’s MedWatch program.
With further questions or more information regarding a reimbursement, people who took the recalled Tydemy can call Inmar Rx Solutions at 866-480-8206 during normal business hours.
Lupin / Food and Drug Administration
Why Are These Birth Control Pills Less Effective?
The Tydemy pill is made up of three ingredients, Thibodeau explained: an estrogen called ethinyl estradiol, a progesterone called drospirenone, and a folate supplement.
Even though she doesn’t personally see Tydemy often in her line of practice, Thibodeau said that there are a number of similar medications with this type of chemical makeup.
There’s no issue with these ingredients—instead, the pills may be missing out on adequate levels of ascorbic acid, which is just another name for vitamin C, Thibodeau said.
The concentrations of vitamin C in the body can impact estrogen levels, she explained.
“The thought is that if vitamin C levels are not the same as what was tested, then there’s concern that the estrogen levels may be different in people taking these pills,” Thibodeau said. “Thus, the efficacy of the birth control may have decreased.”
In addition to the risk of unexpected pregnancy, people who were taking recalled Tydemy might also experience bleeding or other menstruation symptoms if the estrogen levels aren’t functioning in the pill properly, Thibodeau said.
But that being said, low levels of ascorbic acid aren’t necessarily a threat to human health, she said, unlike some other medication recalls in which medications can be actively risky for people taking them.
Related: The Best Birth Control Options To Consider for Your 20s and Beyond
Getting Contraception That Works
Though the recall is precautionary, it’s important that people check the specific lot code of the Tydemy they’re taking so that they can get access to fully effective pills as soon as possible.
People can check with their doctor or pharmacy to see which specific lot of Tydemy they were prescribed if they don’t have the packaging on hand, Thibodeau said.
If someone was taking the recalled medication, it should be fairly easy to switch to something that works.
“Their doctor can either prescribe an equivalent contraceptive,” she said. “There’s no problem in switching birth control pills if they’re equivalent—the next day when you’re due for your pill, you just take the new prescription.”
According to Thibodeau, Yasmin or its generic forms have the same estrogen and progesterone that Tydemy does, and people can take a folate supplement separately—that would be an equivalent option people could switch to.
If people are interested, they could also try to get in touch with their pharmacy to see if they could simply get Tydemy with a different and non-recalled lot code, Thibodeau added.
“[Healthcare providers] should be able to offer a pretty easy solution,” she said.
Related: What Is Opill? FDA Approves First Over-the-Counter Birth Control
For more Health news, make sure to sign up for our newsletter!
Read the original article on Health.

