There’s a New Reason for Athletes to Consider Muscle-Oxygen Sensors
This article originally appeared on Outside
One of the notable topics that popped up at the American College of Sports Medicine conference earlier this month was muscle oxygen monitoring. (For more from the ACSM conference, see my previous updates on supershoes and sports psychology.) I wrote an article last year on muscle oxygen sensors, wondering if they would turn out be "the next great fitness wearable." They're not there yet, but the new research at ACSM and elsewhere is attempting to build a case.
Quick background: muscle oxygen sensors for athletes are matchbox-sized devices that you stick onto a relevant muscle. For runners or cyclists, that's somewhere on your legs. The device uses near-infrared spectroscopy to assess what fraction of the hemoglobin and myoglobin molecules in the tissue are carrying oxygen. If the number is increasing, it means that your heart and lungs are delivering more oxygen than the muscle is using. If it's decreasing, it means demand exceeds supply and exhaustion awaits. The main player in the muscle oxygen space is Moxy, whose devices currently sell for $879.
The new results at ACSM come from a group led by Brett Kirby at Nike, working with other key members of Nike's Breaking2 team: former colleague Brad Wilkins of the University of Oregon, plus Ida Clark, Anni Vanhatalo, and Andrew Jones of the University of Exeter. And the question it addresses is very relevant to the pursuit of a two-hour marathon: is muscle oxygen still a useful metric after you've already been exercising for two hours?
Back in 2021, Kirby and colleagues published data showing that muscle oxygen measurements can reveal when you've crossed a "critical metabolic rate," and how much time you have left before exhaustion once you've crossed it. The new data compares quadriceps muscle oxygen data taken during all-out three-minute sprints when you're fresh to similar data taken after two hours of moderately hard cycling. The absolute numbers are different: you actually maintain higher muscle oxygen levels when you're exhausted, presumably because other sources of fatigue prevent you from digging as deep during the sprint. But the muscle oxygen trends--how quickly it's increasing or decreasing--still accurately predict when you'll give up. That's a big step toward demonstrating that muscle oxygen might be useful in the field, not just the lab.
Another new study, this one published earlier this month in the European Journal of Applied Physiology, makes the case that muscle oxygen sensors might be able to replace cumbersome lactate testing in some contexts. It was led by Philip Batterson of Oregon State University (and also includes Kirby and others as co-authors). Lactate testing has been used in exercise science labs for decades, and in recent years portable lactate monitors requiring a drop of blood from a finger or ear prick have grown in popularity. After I wrote about the "Norwegian Method" of double threshold training, which relies heavily on lactate testing to maintain the appropriate intensity, the main question I got was how to implement this sort of training without the hassle of repeated lactate testing. I didn't have a great answer.
The new study essentially administered a standard lactate testing protocol, alternating three minutes of running with 30 seconds of rest (during which the blood was collected for lactate testing), with the intensity of the running stages ramping up until the subjects reached exhaustion. This sort of test produces a characteristic lactate curve, from which you can calculate two distinct thresholds: LT1, where lactate first begins increasing from its resting values, and LT2, sometimes called lactate turnpoint, where it increases more sharply and will never stabilize even if you keep the pace the same. (For more on the admittedly confusing terminology of thresholds, see this deep dive.)
During the lactate test, the subjects also wore Moxy monitors on their quadriceps (and elsewhere, which we'll ignore). The key finding is that muscle oxygen produced a curve just like the lactate curve, with two identifiable thresholds that were statistically indistinguishable from the lactate thresholds. This suggests that a muscle oxygen monitor, which requires no bloodletting or inconvenient breaks in your workout, might be able to stand in for lactate testing.
There are some caveats. Most notably, it's not the actual value of muscle oxygen that gives you the information you need. Instead, it's the trend: how quickly it's increasing or decreasing. And there's another catch: whenever you rest, muscle oxygen increases sharply. Then when you start exercising again, it decreases sharply before settling down to a more steady slope. So the analysis in the new study (and previous studies) ignores all readings during the first 60 seconds of each exercise stage, then calculates the slope from the subsequent data.
Here's some sample data from one of the incremental tests. The dots show muscle oxygen readings. Whenever the curve is increasing sharply, those are the 30-second rest periods. When it's decreasing sharply (red lines), that's the data that's omitted. The "real" slopes are shown with orange lines:
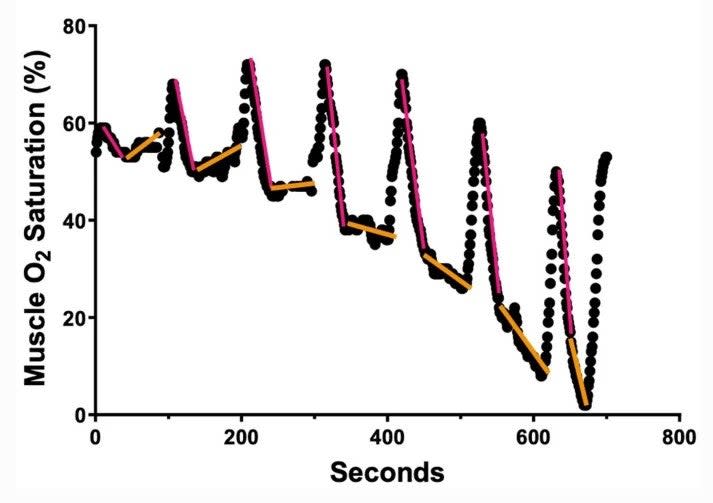
The key thing to notice here is that at the lower intensities early in the test (on the left), the orange slopes point upward. That means oxygen supply is greater than demand in the muscle. At the higher intensities later in the test (on the right), the slopes are progressively steeper downward. That means demand exceeds supply. The critical metabolic threshold, where the orange line would be horizontal, would be at a pace somewhere between the third and fourth stages. Plotting the slopes of the orange lines on a separate graph gives you the equivalent of a lactate curve, and should enable you to use real-time muscle oxygen readings to stay at a desired intensity level if you decide to try training Norwegian-style.
There's still a lot of math going on here. When I spoke to researchers in this space last year, the general sentiment was that muscle oxygen offered useful insights, but that the challenge would be making those insights accessible (not to mention affordable) to the average consumer. I think that assessment remains true for now, but you can start to see glimmers of what a hypothetical consumer-friendly muscle oxygen sensor might look like in the future--and, more importantly, why you might want one.
For more Sweat Science, join me on Twitter and Facebook, sign up for the email newsletter, and check out my book Endure: Mind, Body, and the Curiously Elastic Limits of Human Performance.
For exclusive access to all of our fitness, gear, adventure, and travel stories, plus discounts on trips, events, and gear, sign up for Outside+ today.

