Nasal flu vaccine, FluMist, may soon be available to self-administer at home
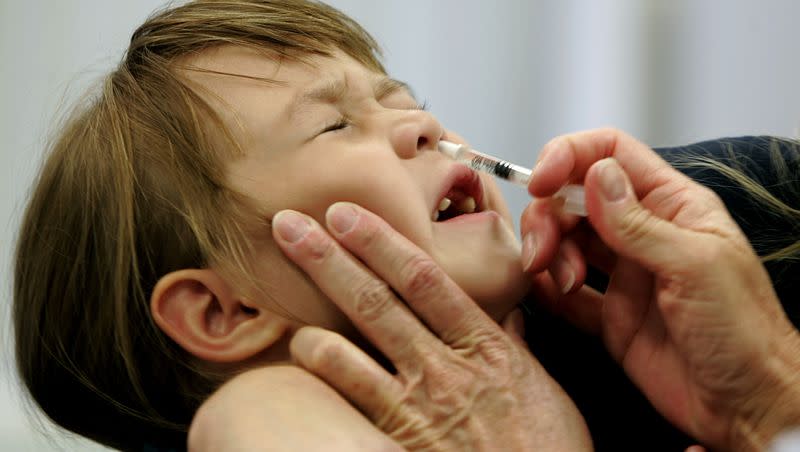
The nasal spray flu vaccine FluMist is under review by the U.S. Food and Drug Administration to become the first and only self-administered flu vaccine, per a press release from AstraZeneca.
Though the nasal vaccine has been an option for vaccine recipients since 2003, AstraZeneca requested on Tuesday for the FDA to approve self-administration for adults ages 18 to 49 and to allow adults to administer the vaccine to children ages 2 through 17, according to CBS.
Lisa Glasser, head of U.S. medical affairs, vaccines and immune therapies for AstraZeneca, told CNN, “One of the things we’ve learned from the pandemic is that actually people can do things for themselves, they can take maybe more responsibility for their own health care in their own hands than perhaps we realized or even thought possible.”
She continued, “we put nasal swab tests in people’s hands and they used them successfully.”
Who needs the flu vaccine?
The CDC recommends everyone 6 months and older get the flu vaccine each year. While only half of Americans typically do get vaccinated against the flu, health authorities are hoping that this new, less painful method will encourage more people to get vaccinated.
In a 2017 study, the CDC found that vaccinated adults had a 52-79% lower risk of death than unvaccinated patients hospitalized for the flu.
The study also found that ICU stays were shorter for vaccinated adults ages 50 and older.
How does FluMist work?
Dr. Carol Cooper explained how nasal flu vaccinations work in a 2012 video for Streaming Well. Nasal vaccines have an awakened form of the influenza virus, she said. This vaccine virus can’t invade the body like normal viruses can, but instead produces antibodies that help the patient resist the real flu.
Injected flu vaccines use either inactivated or genetically engineered viruses. The CDC reported, “Inactivated vaccines are not live and cannot replicate. These vaccines cannot cause disease, even in an immunodeficient person.”
Live attenuated vaccines and inactivated vaccines have different results in the body. Inactivated vaccines mainly produce antibody production, while live vaccines create immune responses that look like natural infection, per the CDC.
Like the name suggests, FluMist is a formula that gets sprayed into the patient’s nose. FluMist’s website explains that the spray begins in the nasal passage and enters the bloodstream to “help circulate antibodies throughout the body.” The vaccine also triggers patients’ cells to help them build a resistance to potential infections.
Side effects may include a sore throat, fever, runny nose, headache, vomiting and muscle aches, per WebMD. Cooper suggested in the 2012 video that nasal flu vaccines are more effective than injectable vaccines for kids and teenagers and more preferred because they’re painless.
Who can use nasal flu vaccines?
Healthy, nonpregnant people from 2 to 49 years of age are approved to receive nasal spray flu vaccines from medical professionals, per the CDC.
People with weakened immune systems shouldn’t use FluMist. WebMD offers this list of people who also shouldn’t take FluMist:
Pregnant women.
Children under 2 years old.
Adults 50 or older.
Children under 5 with asthma.
Anyone with allergies to ingredients in the flu vaccine.
People with chronic heart or lung diseases.
Those with diabetes or kidney failure.
Anyone who has had Guillain-Barre syndrome.
Why was the nasal flu vaccine temporarily stopped?
NBC reported in 2018 that the nasal flu vaccine had been off the market for two years since it “barely worked against one common strain of flu in kids.”
The Advisory Committee on Immunization Practices did not endorse the vaccine in 2016 and 2017 since it “only reduced the risk of getting influenza by 3% over the past three flu seasons,” per NBC. However, eMPR reported that ACIP voted to renew their recommendation of FluMist for the 2018-2019 flu season.
FluMist’s website notes that, during the current flu season, ACIP hasn’t made a specific recommendation for which flu vaccine to get.

