Material World: No PFAS, No Problem

Material World is a weekly roundup of innovations and ideas within the materials sector, covering news from emerging biomaterials and alternative leathers to sustainable substitutes and future-proof fibers.
Impermea Materials
Impermea Materials CEO David Zamarin founded the company nine-and-a-half years ago when he was a high-school sophomore.
More from Sourcing Journal

Zamarin was inspired to start the advanced materials company because he wanted to keep his expensive Jordan sneakers clean. What was on the market at that time—unbeknownst to Zamarin—were cleaning agents full of PFAS that required suiting up in protective gear, complete with respirator mask, in order to apply the solution.
Zamarin was enrolled part-time taking chemistry classes at the University of Pennsylvania when he was “asking really naïve questions” about natural alternatives to these toxic solutions. Research led him to discover “what’s known as the ‘lotus leaf effect,’ which is what all these chemistries try to mimic,” he said, describing eight months of developing a “minimal viable product” he launched in early 2014.
While Impermea looks a lot different from its origin story, it’s still focused on finding natural alternatives to C6 chemicals.
Until a 2016 class-action lawsuit against Dupont thrust PFAS into the mainstream, ” no one really knew what PFAS was,” Zamarin said, describing the challenge of finding a sustainable option that wouldn’t compromise performance, even though “in reality we did” in the earliest days.
“And we realized that the industry just didn’t want that,” he said, going on to explain that “No one was willing to sacrifice performance in exchange for greater sustainability, even though there was pending litigation or regulation coming out.”
Impermea Materials has worked to identify the unique molecules to “functionalize ordinary substrates to perform in extraordinary ways” with coatings that offer liquid repellency as well as resistance to stains, fading and mildew. These molecules are also PFAS-free, 100 percent fluorine free, non-toxic, water-based, and are as recyclable, compostable and repulpable as the substrate they’re applied to. When applied to textiles, the water-based coating technology creates a superoleophobic and superhydrophobic repellent coating for various fabrics, ideal for weather gear and menstrual underwear.
Hydro-Tex 1000 is powered by Siloalkoxyurysilane, a water-based technology designed to develop a breathable, durable, superhydrophobic repellent textile coating. It’s a safe, non-toxic, fluorine-free treatment optimized for use on cotton, linen, rayon, polyester, denim, nylon and spandex, among others. Hydro-Tex + UV 1010 is a water-based technology designed to develop a breathable, durable, superhydrophobic repellent coating for textiles that also blocks harmful UV rays. Hydro-Tex + Virushield 1030 is Impermea’s patent-pending protective coating technology designed to turn a conventional textile into a breathable, superhydrophobic barrier that repels larger droplets and traps smaller droplets that may prevent transmission by containing active viruses.
Apple and Meta have already come knocking, Zamarin said of interest in Impermea’s technologies. “[I]t’s been tough to penetrate the textile market; it’s one of the most fragmented business models I have ever seen,” he said.
Cornell University
The fashion industry is trying to reduce how much waste it generates. But what about the existing waste?
“We have to face the reality: this waste is already generated and it’s not going anywhere,” Juan Hinestroza, a professor of fiber science and apparel design as well as director of the Textiles Nanotechnology Laboratory in Cornell University‘s College of Human Ecology, said. “We need to find ways to tackle this.”
Researchers at Cornell University have found a way to chemically break down clothing and reuse polyester compounds to create fire-resistant, antibacterial or wrinkle-free textile coatings. The proof-of-principle study (which uses a method known as controlled crystallization) was partly funded by the National Science Foundation and should inspired the fashion industry, which generates 20 percent of global solid waste—much of which ends up illegally dumped in other countries, which Hinestroza has seen firsthand.
“I went to some countries and I saw a lot of textile garbage that was supposed to be recycled or donated, and I was concerned about that,” he said. “I wanted to see if we could reuse these materials into something that will have more value.”
That’s where polymerization comes in.
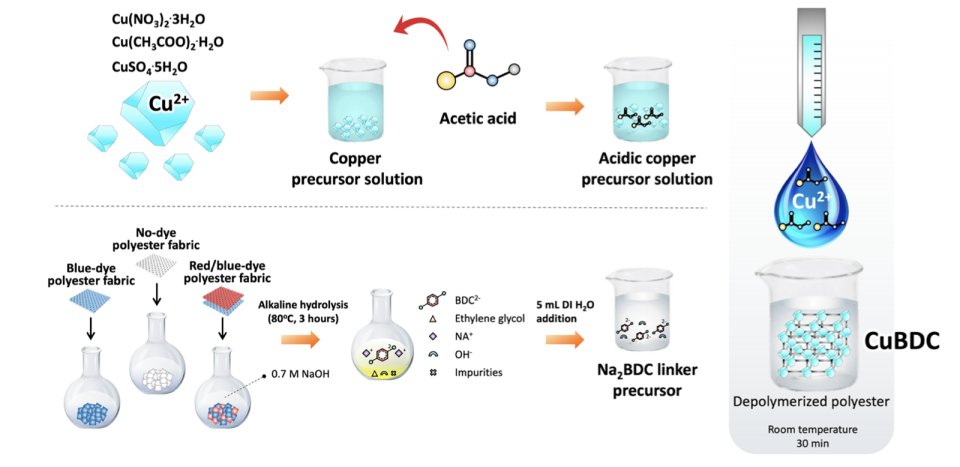
A journal study describes the process of cutting up textiles and chemically decomposing them into a “soup” of raw materials, dyes, additives, dirt and esters. A metal solution is added to that soup and building blocks from the polyester share an affinity with the metal, selectively linking together metal compounds to form tiny cages, also known as metal-organic frameworks, that settle at the bottom of this so-called soup.
“These metal organic frameworks are like detectives; they can find a specific molecule you’re looking for no matter what’s around it,” Hinestroza said. “Initially, people didn’t believe it worked. But we tested it, and the trick was to encapsulate a metal into a cloud or a droplet of [an] acid environment. And then hopefully, minimize the amount of pollution that’s been generated.”
These cages are then used to make coatings, though the coatings may need structural tweaks to tailor to individual uses (wrinkle resistance, antibacterial).
“My ultimate goal is to create a universal finish,” he said. “In academia, we are allowed to have these dreams.”
“Upcycling of Dyed Polyester Fabrics into Copper-1, 4-Benzeedicarboxylate Metal-Organic Frameworks” was published in the Industrial & Engineering Chemistry Research journal in late March. Phillip Milner, assistant professor of chemistry and chemical biology in the college of arts and sciences at Cornell, and Tyler Azbell, a doctoral student in Milner’s laboratory, co-authored the study.

