Artificial Pancreas: What to Know
Medically reviewed by Kelly Wood, MD
An artificial pancreas can make a big difference in the day-to-day life of a person with type 1 diabetes. The Food and Drug Administration (FDA) has approved several versions. They mimic the work that the pancreas does (automatically checking blood sugar levels and adjusting insulin doses accordingly).
Type 1 diabetes is an autoimmune condition in which the body attacks the insulin-producing cells in the pancreas. Insulin is a hormone that helps regulate blood glucose (sugar) levels.
This article covers everything you need to know about an artificial pancreas, including how it works, which systems are available, costs, side effects, and future systems.
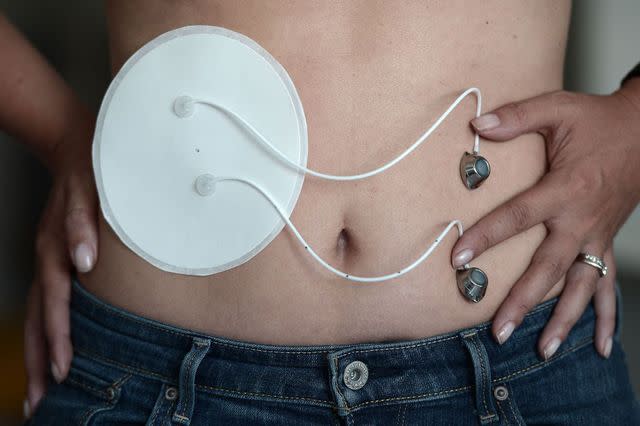
FREDERICK FLORIN / Staff / AFP / Getty Images
How the Pancreas Works
The pancreas is a glandular organ located inside the abdomen behind the stomach. It is part of the endocrine system (glands that make hormones). One of the roles of the pancreas is to help maintain healthy blood sugar levels. It does this by producing and secreting hormones, including insulin and glucagon.
For example, when you eat foods containing carbohydrates, your blood sugar levels rise. In response, the pancreas sends insulin to help move glucose (sugar) from the blood to different cells within the body (muscle, fat, or liver) to be stored or used as energy. This causes blood sugar levels to lower.
The pancreas also produces glucagon. Glucagon’s job is to raise blood sugar levels when they become too low. It does this by converting stored glucose in the liver and putting it back in the bloodstream or stopping glucose from being taken from the blood to be stored in the liver.
Glucagon can also help make glucose from other sources in the body. Insulin and glucagon work together to keep blood sugar levels in a healthy range.
In addition to producing hormones, the pancreas makes and secretes digestive enzymes.
How an Artificial Pancreas Works
An artificial pancreas, also known as an automated insulin-delivery (AID) system, hybrid closed-loop system, or bionic pancreas, is designed to mimic the work of a healthy pancreas to keep blood sugar levels from rising too high.
It automatically and frequently checks blood sugar levels day and night. Depending on the blood sugar level readings, the artificial pancreas system will adjust and deliver insulin doses via an insulin pump to bring blood sugar levels down and into a healthy range.
An artificial pancreas is made up of three devices:
A continuous glucose monitor (CGM) monitors blood glucose levels every few minutes using a tiny sensor inserted under the skin. The CGM sends the blood glucose level readings to a program installed on a smartphone or insulin pump.
The controller, or computer program, takes the blood glucose readings and uses an algorithm (set of rules and problem-solving operations) to calculate how much insulin is needed to bring blood sugar levels back to the target range. It sends this information to the insulin pump.
The insulin infusion pump adjusts and delivers small doses of insulin throughout the day in response to blood sugar levels.
What Automated Insulin Delivery Systems Are FDA-Approved?
The Food and Drug Administration supports the development of new medical devices while ensuring they are safe and effective. The FDA has been collaborating with researchers, diabetes patient groups, diabetes care providers, and medical device manufacturers to further the development of AID systems.
The FDA approved the first hybrid closed-loop system on September 28, 2016. Since then, several AID systems have received FDA approval. FDA-approved artificial pancreas systems include:
Medtronic MiniMed 670G (approved in 2016)
Medtronic MiniMed 770G (approved in 2020)
Medtronic MiniMed 780G (approved in 2023)
Tandem Control IQ (approved in 2019)
Insulet Omnipod 5 (approved in 2022)
iLet®Insulin-Only Bionic Pancreas System (approved in 2023)
The FDA has also approved the Tidepool Loop in early 2023. It is an app used to automate insulin dosing.
Who’s Developing AID Systems?
Several manufacturers are developing AID systems with different features and options to accommodate individual needs. They work with a variety of insulin pumps and CGMs. Talk with your diabetes care team to see what AID system might be best for you.
Medtronic Diabetes
Medtronic Diabetes has three AID systems available for purchase by prescription:
Medtronic MiniMed 630G is for people with type 1 and type 2 diabetes ages 14 and over.
Medtronic MiniMed 770G is for people with type 1 diabetes ages 2 and over.
Medtronic MiniMed 780G is for people with type 1 diabetes ages 7 and over.
Tandem Diabetes Care
Tandem Diabetes Care offers its Control IQ technology, which automatically adjusts insulin levels based on Dexcom G6 CGM readings. The Control IQ technology is integrated into the tandem t:slim X2 insulin pump. It is approved for people with type 1 diabetes who are age 6 and older.
Omnipod 5 From Insulet Corp
The Omnipod 5 System is the only tubeless automated insulin delivery system. It is based on Dexcom G6 CGM readings to manage blood glucose. It is completely controlled by a compatible smartphone. It is intended for people with type 1 diabetes ages 2 and over.
How Much Does a Closed-Loop System Cost?
How much an artificial pancreas costs depends on insurance coverage and which closed-loop system you choose to purchase. Insurance might not cover all the costs of an AID system but may be able to significantly reduce the price. For example, some insurance companies will cover 80% of the cost, leaving you to pay 20%.
Aside from the initial cost of purchasing a closed-loop system, the person will need to pay for an insulin pump and CGM supplies on an ongoing basis.
An insulin pump costs about $6,000 without insurance. Depending on which CGM you choose, the cost may be as low as $40 per month with insurance. However, even with insurance, the yearly cost of supplies for an AID system may be between $3,000 to $6,000 per year or more if you don’t have insurance.
Most manufacturers have cost calculators on their website to help determine the cost depending on your insurance plan or if you are cash pay. Some manufacturers also offer free trials, coupons, savings programs, or financial assistance to help keep the costs down.
Additionally, some AID system components are health savings account (HSA) or flexible spending account (FSA) eligible.
Artificial Pancreas Side Effects
Use of an artificial pancreas is generally considered to be safe. Many users report successful and effective use of artificial pancreas systems to help in managing blood sugar levels. However, there is potential for some risks or side effects associated with use, including:
Low blood sugar (hypoglycemia)
High blood sugar (hyperglycemia)
Red, irritated skin around the infusion patch
Future AID Systems
Many companies and manufacturers are working to develop new AID systems. With more and more companies putting efforts into the innovation and research of these devices, the future is full of potential for improved care and treatment options for people with diabetes.
Companies working to develop new AID systems include:
Bigfoot Biomedical
Beta Bionics
Diabeloop
Dose Safety
DreaMed Diabetes
EoFlow
Lilly Diabetes
Pancreum
Tidepool Loop
TypeZero Technologies
Do-It-Yourself AID Systems
Some technology-savvy people in the diabetes community with a good understanding of how to treat their type 1 diabetes have developed do-it-yourself (DIY) AID systems. They developed these DIY programs out of frustration for the slow pace of technology advancements in diabetes care and management.
These systems are open-source apps available for free that adjust insulin delivery for people with type 1 diabetes. While DIY AID systems have gained popularity over the years, some users have chosen to use one of the several commercial AID systems now available.
The DIY AID systems basically hack your insulin pump with the open-source app to make it communicate and work with your CGM device. They are often more customizable than commercial AID systems.
Tidepool Loop is an app that originated from a crowdsourced diabetes solution, expanding off the DIY AID system movement. It is FDA-approved and allows the user flexibility to use other diabetes devices of their choice.
It’s important to keep in mind that most DIY AID systems are not FDA-approved and do not have formal technical support. This may make it difficult for someone to set up or troubleshoot if glitches occur.
A 2023 study involving 77 adults with type 1 diabetes compared DIY AID systems to commercial AID systems. The researchers concluded that DIY AID systems were non-inferior to commercial AID systems in regards to blood sugar time in range. This means they performed about as well as the commercial AID systems.
If you are interested in using a DIY AID system, talk with your healthcare team before making any changes to your diabetes management plan.
Summary
An artificial pancreas, also known as an automated insulin-delivery (AID) system, hybrid closed-loop system, or bionic pancreas, is designed to mimic the work of a healthy pancreas to keep blood sugar levels from rising too high.
An artificial pancreas is made up of three different devices: a continuous glucose monitor (CGM), a controller or computer program, and the insulin infusion pump. AID systems are generally safe and effective. However, side effects may include low blood sugar, high blood sugar, and red, irritated skin around the infusion patch.
Several FDA-approved AID systems are available, and many more companies are developing new AID systems. Some people use do-it-yourself AID systems using open-source apps.
The cost of using an AID system depends on the system you use and if you have insurance. The average initial cost will vary depending on insurance coverage, with a yearly cost between $3,000 to $6,000 for additional supplies. Do-it-yourself AID systems use open-source applications and are more customizable but are not FDA-approved and lack formal technical support.
Read the original article on Verywell Health.

