113 Women File Lawsuit, Allege Birth Control Mix-up Led to Unwanted Pregnancies
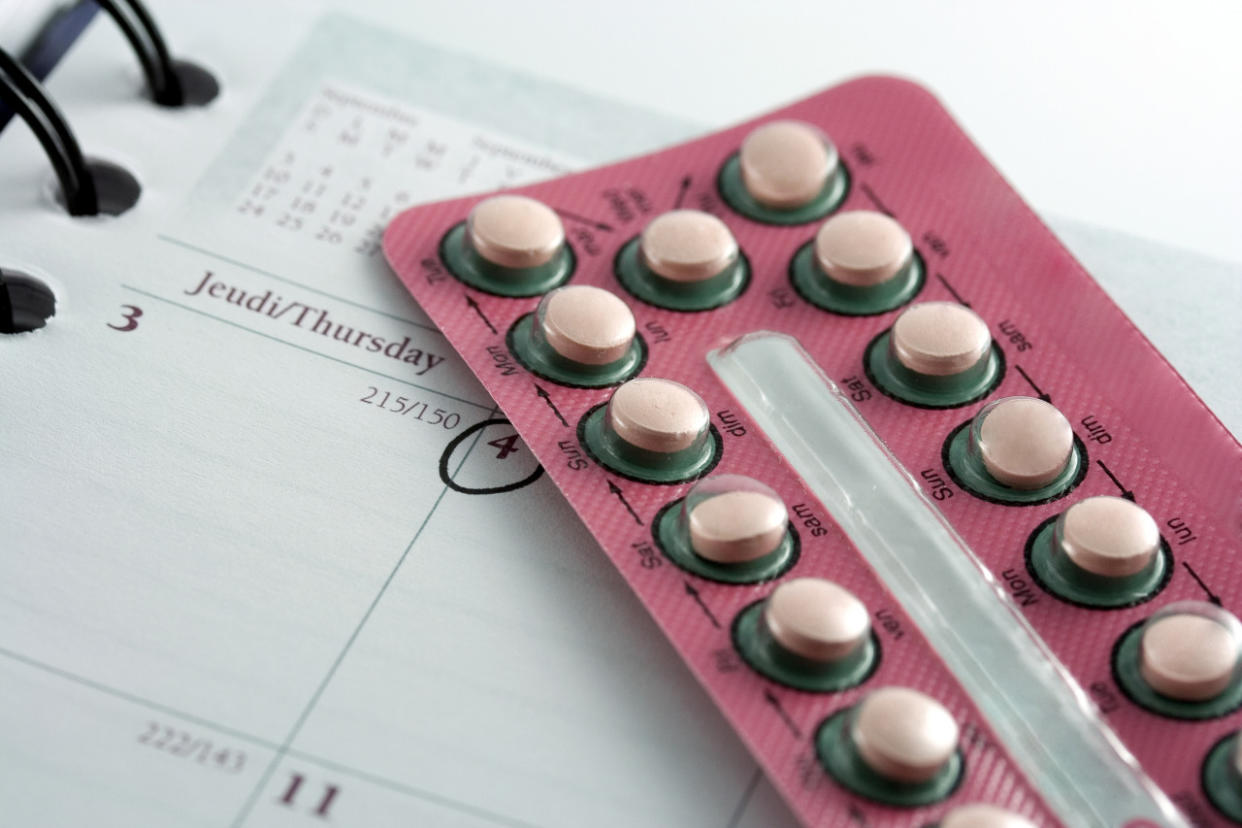
Because birth control pills are meant to be taken in specific order, the reversed-pill packages put women at risk for unintended pregnancies. (Photo: iStock)
In 2011, a Kansas City woman noticed the pills in her prescription for Cyclafem 7/7/7 were flipped, reversing the order. In response, the FDA recalled 3.2 million blister packs of the birth control from Irish drug manufacturer Endo Pharmaceuticals’s subsidiary Qualitest Inc.
However, the damage was already done, according to a lawsuit filed in Philadelphia last week by women who say they conceived as a result of the mix-up. The documents state that 113 pregnancies in 26 states resulted from the birth-control blunder, with 94 women carrying to term, says philly.com.
Because birth control pills are meant to be taken in specific order, the suit alleges that the reversed-pill packages exposed these women to unintended pregnancies. The women are now seeking millions of dollars in damages for pain and suffering, medical costs, and the cost of raising the children.
In addition to Cyclafem 7/7/7, some of the birth control brands recalled due to incorrect packaging were Cyclafem 1/35, Emoquette Gildess FE 1.5/30, Gildess FE 1/20, Orsythia, Previfem, and Tri-Previfem.
In a 2012 Atlantic report, the suit’s lead attorney, Keith Bodoh, emphasized the “heart-wrenching” damages the mix-up caused his clients. Among them are two 17-year-old women who became pregnant, one who dropped out of law school, another who gave up nursing school, and yet another who carried twins.
Bodoh said that Qualitest’s slow response time in realizing the error was irresponsible. “When they sell the pills to the public, they’re supposed to have adequate safety measures in place so that no defective products reach the shelf,” the attorney told The Atlantic. “But it makes it more egregious when you learn that the products were on the shelf for at least 10 months in some cases.”
According to Buzzfeed, a class-action lawsuit filed in Georgia on November 4. But because only 53 blister packs among the half-million returned were found to be defective, a federal judge ruled that the case wasn’t suitable as a class-action suit. Lawyers will have to build their cases to reflect individual women, the symptoms, and any additional evidence that each took a defective set of pills that resulted in a pregnancy.
Attorneys refiled the suit in Pennsylvania, where Endo Pharmaceuticals’s headquarters is stationed.

