Scientists Turn CO2 Into Ethanol With the Power of the Sun

Our atmosphere has too much carbon dioxide, and we need to find a way to get rid of some of it. That's a two-part challenge, because it means we must figure out how to not only pull carbon dioxide out of the air but also a way to store it afterward.
The latest possible solution comes from a group of scientists at Lawrence Berkeley National Laboratory, who have found a way to turn atmospheric CO2 into ethanol using nothing more than the power of the sun. That ethanol can then be safely stored to keep it out of the atmosphere or used as an alternative to fossil fuels.
"As rising atmospheric CO2 levels change Earth's climate, the need to develop sustainable sources of power has become increasingly urgent," said study author Joel Ager. "Our work here shows that we have a plausible path to making fuels directly from sunlight."
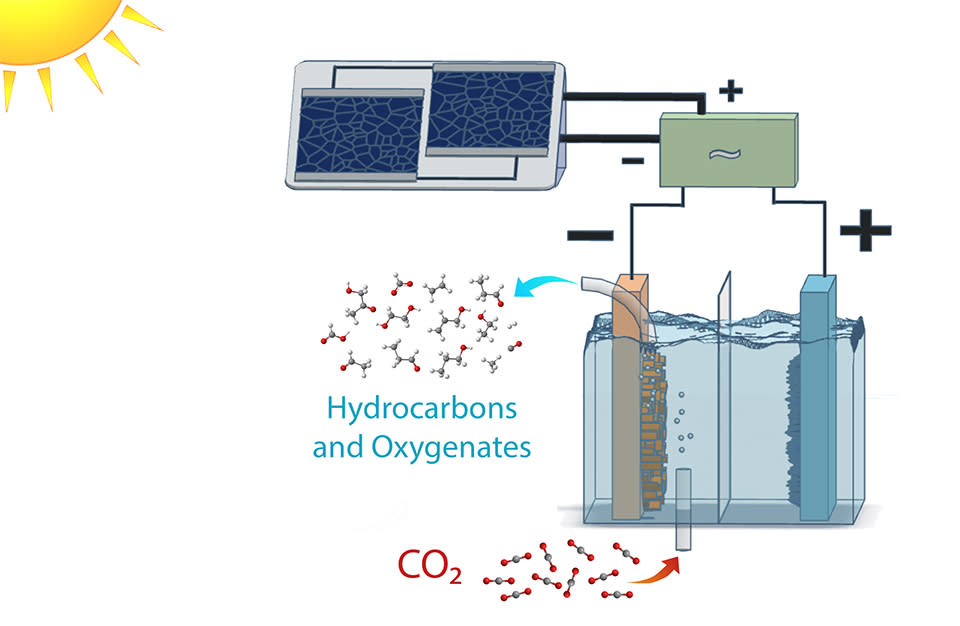
The researchers are part of Berkeley Lab's Joint Center for Artificial Photosynthesis, which seeks to recreate the process by which plants turn sunlight, water, and CO2 into energy. Previous research has found ways to do this, but these methods typically require lots of electricity. The Berkeley researchers were looking to accomplish this with as little electricity as possible to make the process as energy-efficient as they could.
For artificial photosynthesis, electricity use is measured by sun illumination. 1-sun illumination is the electricity generated when the sun is directly overhead on a cloudless day, while 0.1-sun illumination would be the energy produced at sunset. Most current artificial photosynthesis machines only work around 1-sun illumination, which is fine in the lab but impractical for the real world, where the Earth's surface isn't always exposed to total sunlight.
To keep the artificial photosynthesis reaction going at low energies, the Berkeley team turned to a variety of new materials. They developed an iridium oxide nanotube anode to turn water into oxygen, and a copper-silver nanocoral cathode to turn the CO2 into ethanol. These materials made artificial photosynthesis possible even at 0.35-sun illumination.
"Reducing CO2 to a hydrocarbon end product like ethanol or ethylene can take up to 5 volts, start to finish," said study author Gurudayal. "Our system reduced that by half."
"This is a big step forward in the design of devices for efficient CO2 reduction," said Berkeley Lab chemist Frances Houle, who was not part of this study. "It provides a clear framework for the future advancement of fully integrated solar-driven CO2-reduction devices."
Source: Berkeley Lab
You Might Also Like

