First Came Blood Sausage, Then Botulism, and Then Botox
What we know today as Botox was lightning in a bottle until it became a docile servant of humankind thanks to Alan Scott who launched his career in medicine with the words, “I wanted to ask questions and find some answers.” But the convoluted story of Botox starts earlier in southern Germany in the late 1700s.
Botox is produced from living matter, meaning it is a biologic which is a naturally occurring substance. Evidence reveals it has existed from the beginning of time as a toxin secreted by the ubiquitous bacterium Clostridium botulinum. Death by poisoning with this toxin was causing epidemics resulting in death in local European populations at a time when their transplanted counterparts were overseas establishing the path for what would become the United States of America. This deadly disease present in Germany was linked to a food. Only those who consumed it were affected while those who did not were spared. The food was blood sausage, and the fatal disease became known as sausage poisoning.
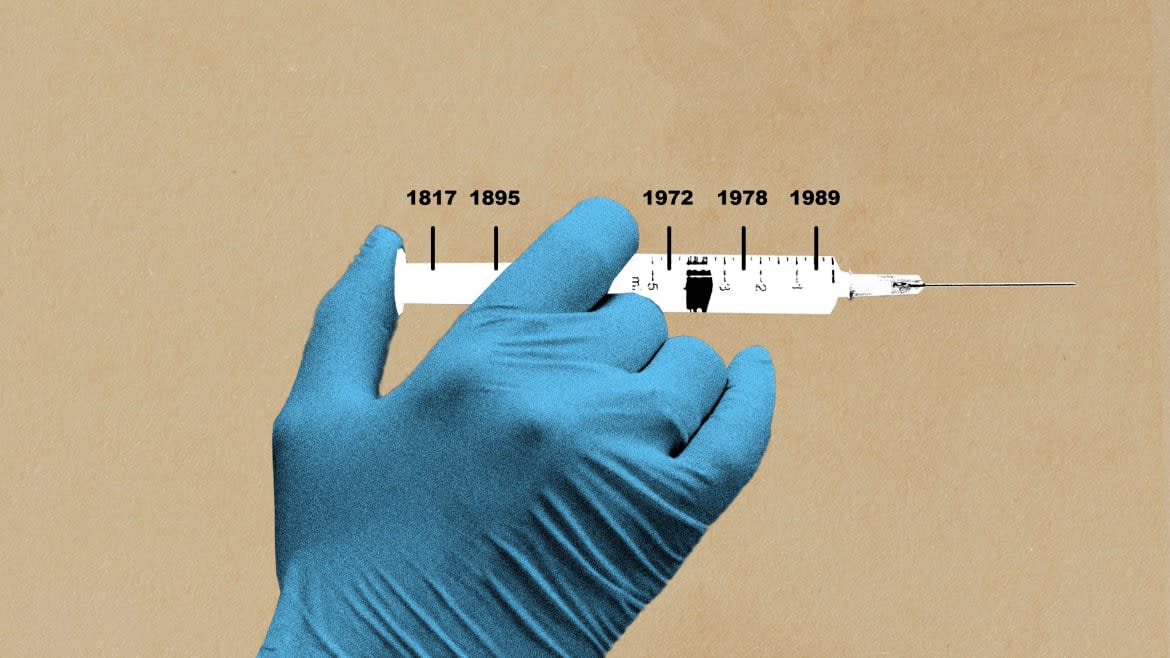
A polymath district health officer acting like a sleuth in 1817 during a recurring epidemic found a fatty substance that was the only common feature in these sausages and selected this substance for study. In his lab he fed it to tiny creatures and all succumbed. When it was inserted in the muscle of a larger specimen, the muscle was weakened but the subjects survived. More curious than cautious, Justinus Kerner then put a drop of fatty poison on his tongue. It caused a tight feeling and dryness of his mouth. This led the intrepid healer to predict the fatty substance could find a use someday as a medicine. How right he was. But it took 150 years and Alan Scott to prove it.
The remainder of the century sausage poisoning recurred sporadically in Europe with the only change being a new name. Substituting the Latin word for sausage, botulis, the disease was now called botulism poisoning. Then in 1895, studying the cause of death of three musicians performing at a funeral that they did not realize would in a way be their own, a biochemist in Belgium named Van Ermengem made a discovery. He identified an otherwise ordinary bacteria that produced a deadly toxin that was later confirmed as the deadliest toxin known. It was death dealing in doses of billionths of a gram.
Can Botox Ease Teeth Grinding?
By the time of World War II, the second such conflict in a generation, concerns about the use of this toxin as a weapon spurred research in an unlikely place. This was in Camp Detrick Maryland in a hastily constructed tar paper shack called The Black Maria. It was named after the structure Thomas Edison built for his experiments making movies. The work in this new make-shift facility was conducted by conscript scientists mostly in their twenties. Acting with urgency in a time of great peril, they isolated the deadly toxin molecule for the first time, created a vaccine ready for use on D-Day and determined the toxin would not be weaponized because it was not suitable for delivery. But the story of the toxin did not end at this time. For the purposes here, it was only the beginning.
One of the researchers, an “old man” of 33, assumed the role of custodian for the culture of bacteria producing the botulinum toxin. Maintaining this culture was legitimate based on a broad interpretation that the toxin was useful in medicine and therefore exempt from a presidential order for the destruction of biological weapons of war.
Dr. Ed Schantz, a lieutenant in the army and later civilian employee at Camp Detrick, remained custodian of the culture for more than 40 years at the newly named Fort Detrick and later the University of Wisconsin Madison. During this time, he provided suitable portions of the toxin to more than 100 researchers in the U.S., Canada, and Europe. In 1972, one of these researchers requesting the toxin was Alan Scott.

Alan Scott
Alan Scott was an ophthalmologist who had started his practice a decade earlier in 1961 after completing training at University of California Berkeley, Stanford, and the University of San Francisco. The launch of Scott’s practice was anything but ordinary. His plan was to work mornings in a laboratory and in the afternoon in a traditional office setting seeing patients. The laboratory he worked in, eventually named Smith-Kettlewell, was established by his mentor Arthur Jampolsky. Alan’s research goal was to find out everything he could about how the extraocular muscles moved the eyes and maintained proper alignment enabling the two eyes to see as one, only better.
Scott’s work in the laboratory with animals, eventually advancing to primates, finally dealt with human subjects. These included consenting inmates at a large correctional institution. This experience proved beneficial for the subjects who received free surgery to correct strabismus. At its conclusion, this experience led Scott to call it one of the most satisfying and productive projects of his research effort to date.
The aim of Scott’s initial study of eye muscles was to eventually arrive at a method to modify their action, achieving normal alignment in a strabismic (misaligned) eye without the need for incisional surgery. His next challenge was to find a suitable substance for weakening a muscle by a predictable amount, producing lasting results, and without harming the patient. An array of agents from alcohol to snake venom proved unsatisfactory. This led him to consider Dr. Schantz’s botulinum toxin, which researcher Daniel Drachman at Johns Hopkins employed to weaken a muscle without causing other damage, even when injected in an embryo.
Now armed with his own supply of Botulinum toxin type A, Scott prepared it for injection by diluting, stabilizing it with human albumin, and freeze drying it. Then he conducted assays determining the dose that would be effective but not lethal in a 20-gram white mouse. With this information in hand, he injected a minute dose of the toxin into the eye muscle of a monkey, and it worked! The weakened muscle changed eye alignment, producing a lasting result. It was the first time something like this had been accomplished, but it also resulted in unexpected dire financial consequences for the project.
In a 1973 report, Scott described the results of these injections. This was good news for science but there were consequences that would surface later. This disclosure made it impossible to patent the process. The idea was in the public domain and unprotected. This made little if any difference to Scott, but for the institutions he would work with, this meant a potential loss of revenue. Lacking a patent, the maintenance of the culture did remain a trade secret and these can be formidable, think of Coca-Cola.
By 1978, and now working with the California Pacific Medical Center, Alan Scott was ready for the next step, an unprecedented move. He would inject the world’s deadliest toxin into a human for the first time. This was momentous and required extreme caution including doing the injection in an operating room, monitoring by emergency personnel, and a stay in the intensive care unit post injection. It went without a hitch, but the minuscule dose required by the Food and Drug Administration (FDA) overseers had no effect. The good news was it caused no harm. According to cautious requirements of the FDA, the dose was increased incrementally in subsequent injections until the desired effect was achieved. Now with safety, effectiveness, and proper dosage established the most unlikely part of Scott’s endeavor began.
At the beginning of the 1980s, Alan Scott would continue working alone as he led a small team in a monumental challenge. He would conduct the demanding process of obtaining FDA approval for a new drug for use in humans. This is carried out in three phases that together determine safety, effectiveness, and other behaviors such as drug interaction and more. The first phase includes tens of patients, the second can include more than a hundred, and the third in the thousands. To accomplish this Scott first worked in-house with his own team. In phases two and three, when the number of subjects reached the hundreds and thousands, he enlisted the aid of hundreds of volunteer doctors to participate.
Because he was working with a biologic, naturally occurring, substance Scott was required by the FDA to have the product produced by a licensed manufacturer. He accomplished this using a firm whose only product was sterile water. In the process, Scott traveled from San Francisco to Albuquerque and assisted with the first two phases of dilution. He was now a de facto but unintended drug manufacturer following rules.
With an ever-increasing staff to manage data and supply, tens of thousands of botulinum toxin vials containing one hundred mouse units (usual doses for a patient were injections between 2.5 and 5 mouse units with a total dose between 25 and 50 units), Scott needed more money. In the beginning, he used his own funds and traded “sweat equity” for laboratory space, but now he needed cash. In a unique move, he gave the hundreds of doctors participating in the trial the opportunity to donate $40 per vial. Most responded, sending $2.5 million. For a safety net, Scott mortgaged his own home in case more was needed. But this was not the solution. Scott had accomplished what he set out to do and tried to sell his company, now called Oculinum. It sounded like a drug name and combined Occu (eye) linum (alignment). He called on more than a half dozen companies, but none wanted to buy the drug. Why? There were two reasons: the market for treating strabismus was small, and the drug had no patent protection. Undaunted, Scott persisted and received FDA approval for Oculinum in December 1989 for use in humans 12 and over for treating strabismus, and blepharospasm.
After establishing a licensing agreement with Allergan Pharmaceutical in 1990, Scott sold Oculinum to the company a year later for $9 million, little more than recouping his expenses and gaining little for his work of 30 years. It is noteworthy that at the time the cost of the effort to obtain FDA approval for a new drug was in the range of $500 million, or 100 times more than Scott spent. Since purchasing Oculinum and re-naming it Botox, Allergan and the company’s successors have sold tens of billions of dollars of the drug, making Botox the leader in the market for this class of drug. In contrast, the stakeholders in the Gatorade trust have received royalties in the range of $1 billion in 30 years.
After this sale, Scott had no further dealings with Allergan and continued his efforts to create more uses for the drug, now called Botox, for treating strabismus. He also treated overacting muscles around the face, neck, and extremities, but his focus was on eye muscles. Scott knew the toxin could smooth wrinkles but said, “I wasn’t interested.” He shared what he knew with other doctors, and nobody made better use of this knowledge than ophthalmologist Dr. Jean Carruthers of Vancouver BC, who with her dermatologist husband, Alastair, launched the cosmetic/aesthetic use of Botox.
Alan Scott sums up his life with Botox like this: “I guess I had all the fun and Allergan made all the money,” and “I was OK in the lab, pretty good in the clinic, but I was a lousy businessman.”

Eugene M. Helveston, MD, is the author of Death to Beauty: The Transformative History of Botox.
Get the Daily Beast's biggest scoops and scandals delivered right to your inbox. Sign up now.
Stay informed and gain unlimited access to the Daily Beast's unmatched reporting. Subscribe now.

