Moderna (MRNA) Begins Dosing in Study on Vaccine Against EBV
Moderna, Inc. MRNA announced that it has dosed the first participant in a phase I study evaluating mRNA-1189, its vaccine candidate against Epstein-Barr virus (“EBV”), one of the most common viral infections in the world.
The randomized, placebo-controlled phase I study called Eclipse will evaluate the safety and tolerability of mRNA-1189 in healthy adults aged between 18 to 30 years. The study will enroll around 270 participants and will be conducted at various sites in the United States. The vaccine candidate, mRNA-1189, is being developed to prevent EBV-induced infectious mononucleosis (“IM”) and potential EBV infection.
Moderna currently has two vaccines in clinical development against latent viruses – cytomegalovirus (“CMV”) and EBV. The company remains focused on developing vaccines against latent viruses like EBV, CMV and human immunodeficiency virus (“HIV”). Currently, there are no vaccines approved to prevent EBV, a major cause of IM.
Shares of Moderna have rallied 87% in the past year against the industry’s decline of 26.9%.
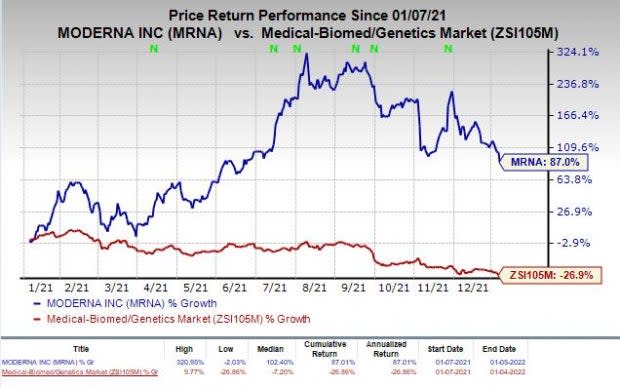
Image Source: Zacks Investment Research
The company is developing several promising mRNA candidates as therapies and vaccines targeting oncology indications and rare diseases.
Moderna is evaluating mRNA-1647 as a CMV vaccine in a pivotal phase II/III study. Like the CMV vaccine candidate – mRNA-1647, mRNA-1189 also contains four mRNAs that encode EBV envelope glycoproteins.
Moderna has several mRNA-based vaccine candidates in clinical-stage development. The company is evaluating three pipeline candidates — mRNA-1893 as Zika vaccine, mRNA-4157 as personalized cancer vaccine and AZD8601 for treating myocardial ischemia — in phase II studies.
The company also plans to begin studies on mRNA-1644 and mRNA-1574 as HIV vaccines and on mRNA-1215 as a vaccine against Nipah virus, shortly.
Moderna’s mRNA based COVID-19 vaccine, mRNA-1273, is approved for emergency use in several countries across the world and has generated billions in sales for the company. In the first nine months of 2021, the vaccine generated sales worth $10.7 billion.
The booster dose of Moderna’s vaccine has also been granted Emergency Use Authorization (“EUA”) for use in adults at least six months after completion of primary vaccination.
Moderna faces stiff competition from Pfizer PFE/BioNTech BNTX, AstraZeneca and J&J JNJ, whose COVID-19 vaccines have also received authorization for emergency/conditional use in several countries.
A booster shot of J&J’s adenovirus-based single-shot COVID-19 vaccine was granted EUA by the FDA in October for adults aged 18 and older at least two months after the primary vaccination with its vaccine or with Pfizer or Moderna’s two-shot mRNA COVID-19 vaccine regimen.
The booster dose of Pfizer/BioNTech’s COVID-19 vaccine has also been granted EUA by the FDA. It is also the only booster which is approved for kids.
Zacks Rank
Moderna currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research