Florida Closes Covid Treatment Sites after FDA Revokes Monoclonal Antibody Authorization
- Oops!Something went wrong.Please try again later.
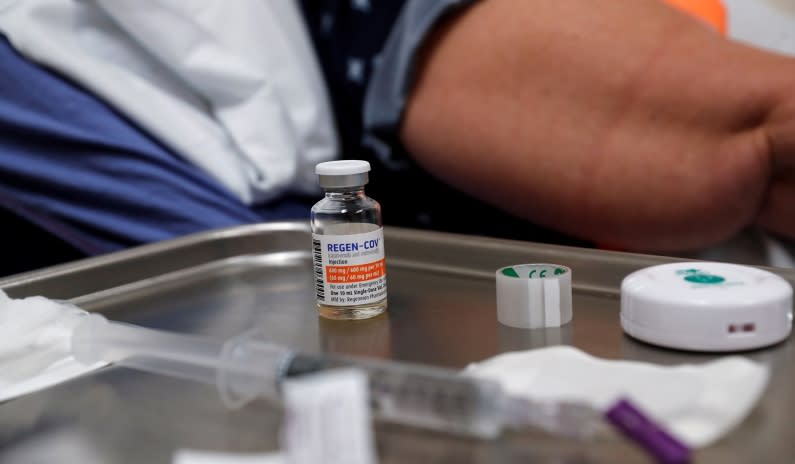
Florida has been forced to shut down sites administering monoclonal antibodies, which have been widely used as supplemental treatment for Covid-19, after the FDA revoked authorization for distribution.
“This evening, without any advanced notice, the U.S. Food and Drug Administration (FDA) revised the Emergency Use Authorization (EUA) for bamlanivimab/etesevimab and REGEN-COV. The revised EUAs do not allow providers to administer these treatments within the United States,” a press release from the Florida Department of Health read.
“Unfortunately, as a result of this abrupt decision made by the federal government, all monoclonal antibody state sites will be closed until further notice,” it continued.
The development comes after Florida surgeon general Joseph Ladapo accused the Biden administration of intentionally restricting the circulation of Covid-19 therapeutics. Governor Ron DeSantis had launched dozens of antibody-treatment sites as part of the state’s strategy to defeat the disease.
The federal government is “actively preventing the effective distribution of monoclonal antibody treatments,” Ladapo wrote in a letter to Secretary of Health and Human Services Xavier Becerra in December.
The Biden administration had reportedly suspended shipments of Covid-19 antibody treatments, which can shorten severity and duration of illness in those who contract the virus, amid mounting evidence that they had been rendered ineffective by the Omicron variant.
Agreeing with Biden that the pandemic has no “federal solution,” Ladapo urged him to allow Florida to conduct its virus management without federal interference the way it deems appropriate for its population, which includes providing treatment alternatives to the vaccine.
“I respectfully request that you allow states and healthcare practitioners to provide treatment options that best benefit the communities they know and serve,” he said.
The press release from Monday emphasized, “Florida disagrees with the decision that blocks access to any available treatments in the absence of clinical evidence. To date, such clinical evidence has not been provided by the United States Food and Drug Administration (FDA).”
It cited a study on the NIH’s website confirming that there have been no definitive clinical conclusions that the monoclonal antibody treatments are ineffective. “…Despite observing differences in neutralizing activity with certain mAbs, it remains to be determined how this finding translates into effects on clinical protection against B.1.1.529,” according to the study.
Over the summer, when DeSantis first promoted Regeneron’s monoclonal antibody treatments, critics, including the Biden administration, saw such a program as a threat to national vaccination efforts.
The Biden administration reversed course in September, admitting the Regeneron treatment’s positive impact, when it purchased “1.4 million additional doses” to “stave off shortages” of the drug as demand exploded, the Washington Post discovered. It also directed the Department of Health and Human Services to set “the rules for distribution.”
Defending against the backlash that he wanted monoclonal antibodies to supplant the vaccine, DeSantis clarified in September that he believed it wise to provide his state with an abundance of resources and treatment options to combat Covid-19. He had said he does not intend to “lean into monoclonals to the detriment of vaccines” or to “play defense with no offense.”
“If you’re at risk,” the governor said, “the best thing you can do beforehand, obviously, is to get vaccinated. But even if you are, and if you’re not, if you do become COVID-positive, you have an opportunity to get early treatment using these monoclonal antibodies.”
“This is not in lieu of [vaccination],” he added. “It’s in addition to.”

