Weight Loss Drugs Zepbound and Mounjaro Are in Shortage Due to 'Demand Increase,' FDA Says
Eli Lilly’s popular weight loss drugs have "limited availability" through the end of April

Eli Lilly
Two popular weight loss drugs are currently in short supply, according to the Food and Drug Administration.
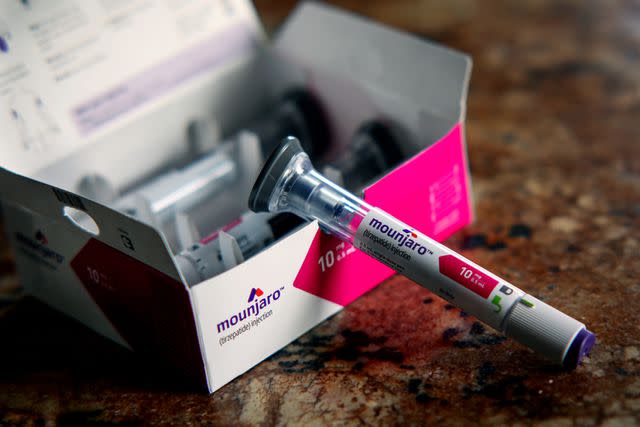
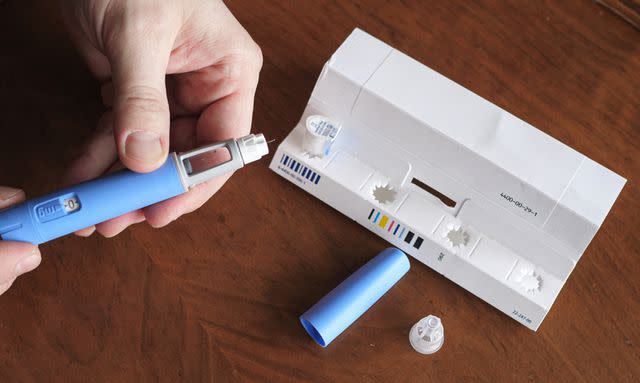
The agency’s website states that currently, two doses of Zepbound and four doses of Mounjaro have "limited availability" through the end of April. Additionally, a “demand increase” is cited as the reason for the shortages.
The two drugs — made by pharmaceutical company Eli Lilly — are brand names for tirzepatide, which has been proven to be highly effective for weight loss by reducing appetite and improving how the body breaks down sugar and fat.
Mounjaro is FDA-approved to treat type 2 diabetes while Zepbound is FDA-approved to treat obesity.
In a statement to ABC News, Eli Lilly said the company is working to replenish its supply amid the “unparalleled surge in demand.”
Sandy Huffaker for The Washington Post via Getty
Never miss a story — sign up for PEOPLE's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from celebrity news to compelling human interest stories.
"We recognize this situation may cause a disruption in peoples’ treatment regimens and are working with purpose and urgency to address it,” a spokesperson for the company told the outlet. “As a medicine company, we know that people rely on us to help reach and maintain their health goals, and we take our responsibility very seriously.”
"While we anticipate intermittent availability in the near term due to unprecedented demand, we expect our investments in manufacturing and supply capacity to progressively increase production of Mounjaro and Zepbound throughout the second half of 2024,” the statement said.
Last month, Eli Lilly released a commercial ahead of the 2024 Oscars criticizing people in Hollywood who are misusing their weight loss drugs.
“We have a point of view about how these drugs are being used,” CEO David Ricks told CNN. “These medicines were invented for people with a serious health condition; they were not invented just to have someone who’s famous look a little bit better.”
Getty
Related: Mounjaro Is 'Significantly' More Effective for Weight Loss Than Ozempic, Study Says
Eli Lilly also released a statement in January emphasizing that they “stand against the use of its medicines for cosmetic weight loss.”
“Mounjaro and Zepbound are indicated for the treatment of serious diseases; they are not approved for — and should not be used for — cosmetic weight loss,” the company said at the time. “Lilly does not promote or encourage use of Mounjaro, Zepbound, or any Lilly medicines outside of a medicine’s FDA-approved indication.”
Many are familiar with Monjaro and Zepbound due to Novo Nordisk’s competing drugs semaglutide, known by brand names Ozempic and Wegovy, which have gained popularity throughout the past year as many have used them off-label for weight loss.
The FDA has also listed four doses of Wegovy as “limited availability” due to demand increases.However, there is no estimated duration of the shortage.
For more People news, make sure to sign up for our newsletter!
Read the original article on People.

