Scientists Are Starting a New Periodic Table
Quantum dots can arrange themselves into artificial atoms and molecules, leading to a new form of chemistry.
Scientists making these atoms in a new, room-temperature process imagine a new periodic table for them.
The dots are nanoscopic semiconductors that emit light, which are popular in electronics research.
Scientists have started a new periodic table for quantum dots, which they refer to as “artificial atoms.” Quantum dots are nano-sized particles that are made of semiconducting materials. Researchers are rapidly exploring new applications for quantum dots, from TVs to lasers to body-nontoxic medical applications.
Like carbon nanotubes, graphene sheets, and classic buckminsterfullerene, quantum dots are considered a nanomaterial—but they also conduct electricity because of the way they trap electrons. This is also why they’re a good candidate for a periodic table of their own. Scientists can arrange them in structures very like naturally occurring atoms of “real” elements.
But quantum dots are themselves made of thousands of atoms, so these artificial quantum dot atoms are relatively huge. Their crystalline format helps them conduct and illuminate, like the pegs you use in a LiteBrite, except the light comes from inside. Different sizes glow in different colors.
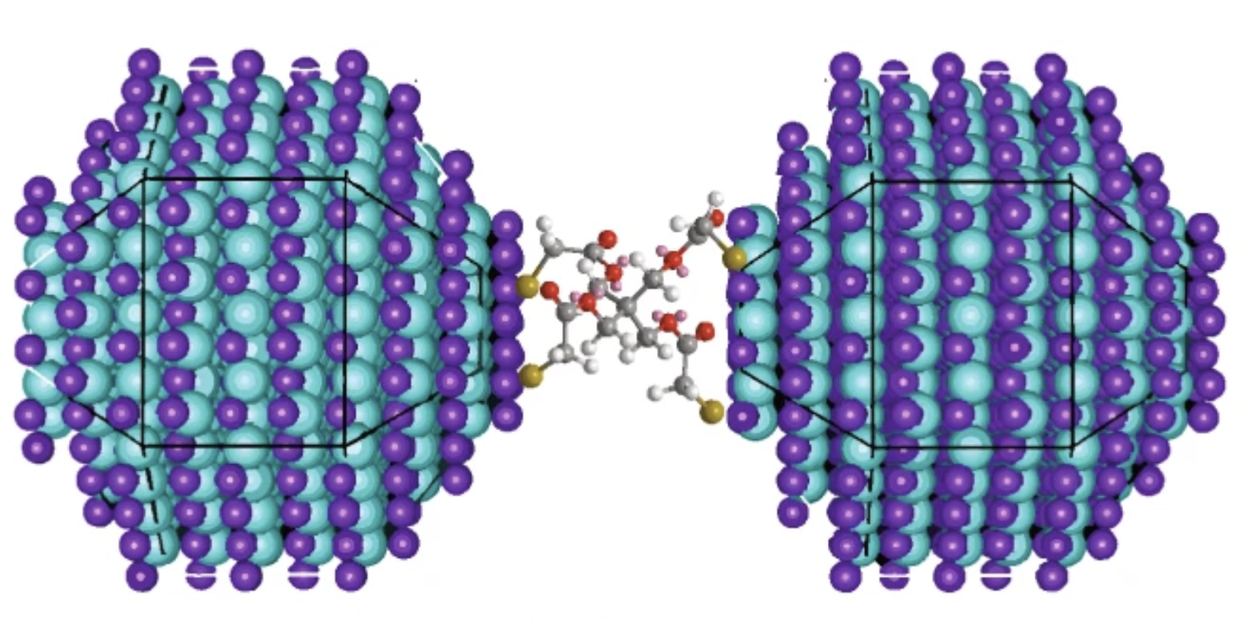
Israeli scientist Uri Banin led the team developing the new periodic table, which he says developed naturally after his group invented a way to allow quantum dots to rearrange and form molecules on their own. “Coupling of atoms is the basis of chemistry, yielding the beauty and richness of molecules,” Banin et al’s evocative abstract begins.
The traditional periodic table accounts for 150 million known molecules made with combinations of the 118 known elements—and now, with a whole new toolbox, who knows what’s next? “Formation of [these colloidal quantum dot (CQD)] molecules sets the stage for ‘nanocrystal chemistry,’” Banin’s team concludes, with the full complement of new entire kinds of molecules that that brings with it. “Such CQD molecules bear significant promise for their utilization in numerous applications, including in light-emitting devices, displays, photovoltaics, and sensors.”
Banin and his team have improved on an existing method for coupling quantum dots into molecular structures. The previous method, molecular beam epitaxy, resulted in larger quantum dots that were less useful and unstable at all but the most extremely cold temperatures. Their new colloidal quantum dot method happens at room temperature, which is another reason for the comparison to the periodic table.
Does it make sense to consider these artificial atoms to be new elements? This is hard to say when the technology is brand new, but the history of science is stuffed with huge paradigm shifts of this nature. In the beginning, scientists doing their absolute best with what they could observe believed that there were very few elements, including “earth,” that were just made of what they appeared to be. They identified different kinds of metals and began associating them and their properties with astrological ideas and mythology.
For a long time, scientists who believed in individual particles they had already begun to call “atoms” were considered heretics. Still, more and more scientists believe matter was made of particles, and long before anyone could really see them using even secondary observations. Maybe in 100 years, the second periodic table will seem as commonplace to people as the existing periodic table is to us today. Certainly, the applications of quantum dots will be legion by then.
You Might Also Like

