Scientists 'accidentally' discovered a way to convert CO2 directly into ethanol

A group of U.S. scientists has discovered a way to turn carbon dioxide, a planet-warming greenhouse gas, into a cleaner-burning motor fuel.
The electrochemical process could be used in waste-to-fuel technologies that transform carbon emissions from coal and natural gas power plants into ethanol, according to scientists at the Energy Department's Oak Ridge National Laboratory in Tennessee.
The team published their findings last week in the journal ChemistrySelect.
SEE ALSO: Here's why the new agreement on 'super greenhouse gases' is a huge deal
Their process turns carbon dioxide into ethanol with little more than extremely tiny spikes of carbon and copper.
Adam Rondinone, the study's lead author and a scientist at Oak Ridge, said they stumbled onto this process nearly by accident.
"We were trying to study the first step of a proposed reaction, when we realized that the catalyst was doing the entire reaction on its own," Rondinone said in an Oak Ridge press release.
"Ethanol was a surprise — it’s extremely difficult to go straight from carbon dioxide to ethanol with a single catalyst," he added.
Rondinone and his colleagues used a catalyst made from carbon, copper and nitrogen and then applied voltage. This triggered a complicated chemical reaction that reversed the combustion process.
Applying these techniques to a solution of carbon dioxide dissolved into water, the scientists produced ethanol with a yield of 63 percent. Typically, this type of electrochemical reaction would result in a mix of different fuels in small amounts, according to Oak Ridge.
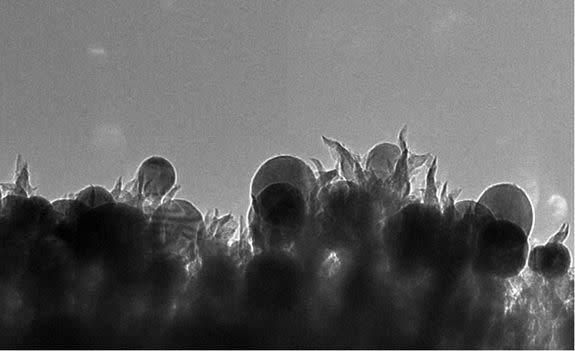
Image: oak ridge national laboratory
The researchers said they plan to further study the catalyst's properties and behavior and will refine their approach to boost the overall production rate of ethanol.
Rondinone suggested the process could be used not only for fossil fuel power plants but also renewable energy systems. He said the excess electricity from wind and solar plants could be stored as ethanol — which grid operators could then burn when the wind wanes or the sun sets.
"This could help to balance a grid supplied by intermittent renewable sources," he said in the press release.
Existing vehicle fleets, and even some aircraft, are capable of using motor fuel containing a mix of ethanol. However, one of the main ethanol production processes in use today, which relies on deriving the fuel from corn, actually aggravates global warming rather than helping to solve it.
