Salt: Defender of the Carotenoids
If flour is the star of bread making, salt is the director, invisible in the dough but controlling its action and timing. Salt adds flavor. It slows fermentation. It tightens gluten and makes the dough less gloppy. And one of its lesser known jobs is protecting the flour’s carotenoids.
The most famous carotenoid, carotene, gives carrots their bright orange color. Carotenoids also contribute color and flavor to flour and bread. (In bleached flour, they have been destroyed.) Some people claim you can watch the color of dough change as you knead, from an orangey hue to a whiter color. What’s happening to the carotenoids? They’re oxidizing. Call in the salt! How can it slow down this oxidation?
First of all, the carotenoids in flour are lutein, lutein monoester, and lutein diester [1]. Lutein is a long hydrocarbon with a carbon ring and a hydroxyl group (an oxygen atom bonded to a hydrogen atom, also called an OH-group) on each end. The OH-group is the reactive part of the molecule.
To make a lutein monoester (below) or diester, one or two fatty acids react with the OH-groups. Each fatty acid also has an OH-group, and it pulls the hydrogen off lutein’s OH-group.The remaining part of the lutein molecule joins the fatty acid, while the lost atoms (O, H, and H) form a water molecule. Lutein monoester still contains one reactive OH-group.
When you start kneading your dough, you work air into it, and air contains oxygen. This oxygen steals hydrogen from the lutein’s OH-group, as well as stealing a hydrogen from the neighboring carbon. (The two stolen hydrogens and the thieving oxygen form water.) Since the lutein’s oxygen now has an extra electron (the electron it formerly shared with the stolen hydrogen atom) as does the robbed carbon atom, the oxygen and carbon turn to each other and form a stable double bond. (Previously they had one bond between them, and they use the extra electrons to form another.)
This oxidized form of lutein is more stable. So as dough is kneaded and oxygen is introduced, more and more oxidized lutein forms.
Enter salt.
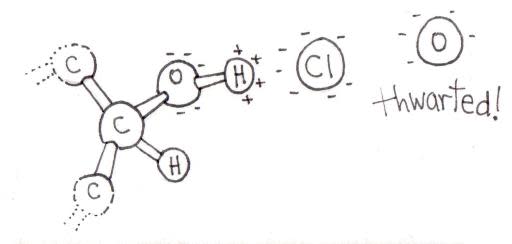
In wet dough, salt dissolves into ions: positive sodium and negative chloride. These ions move around, and, if they encounter an opposite charge, they are attracted to it.
Lutein’s reactive OH-group has a slightly negative charge on the oxygen atom and a corresponding positive charge on the hydrogen atom. (The bigger oxygen hogs the shared electrons, which are more attracted to the big oxygen nucleus than to the weeny hydrogen nucleus.) The hydrogen’s slight charge normally attracts incoming oxygen atoms, resulting in oxidation. But if a negative chloride ion is nearby, it shields the hydrogen and prevents oxidation.
Thus salt allows you to knead your dough without losing all that lutein, and its attendant color and flavor, to oxidation. And that’s worth a standing ovation.
——-
[1] Lepage, M. and R.P.A. Sims, “Carotenoids of Wheat Flour: Their Identification and Composition,” Cereal Chemistry 45 (November, 1968) 600-604. Read it online: http://www.aaccnet.org/cerealchemistry/backissues/1968/chem45_600.pdf
Follow Scientific American on Twitter @SciAm and @SciamBlogs. Visit ScientificAmerican.com for the latest in science, health and technology news.
© 2013 ScientificAmerican.com. All rights reserved.

