Novavax shares pop after clinching COVID vaccine supply deal with European Union
Shares in Novavax soared again in premarket trading on Thursday after clinching a key COVID-19 supply contract with the European Union.
On Wednesday, the European Commission gave the green light to the Maryland-based biotech firm to secure up to 200 million doses, with the first batch of the two-shot vaccines ready for shipment ahead of the winter. But there’s still a big “if” hanging over the deal.
The EU will buy 100 million Novavax doses this year, and it has the option to purchase another 100 million doses through 2023 as soon as the vaccine is approved by the European Medicines Agency. On Wednesday, shares in Novavax surged 18.69% following the announcement, and are up a further 3.6% in premarket trading today.
The EC said in a statement that the contract expands the member nations’ portfolio of vaccines produced in Europe to include one more protein-based vaccine, representing “another key step towards ensuring that Europe is well prepared to face the COVID-19 pandemic."
The Novavax vaccine has been highly anticipated after some big trial wins. Novavax was 89.3% effective in a U.K. and South Africa study conducted in January, 96.4% effective in a large-scale Phase III trial conducted in the U.K. in March, and 90% effective overall in a late-stage study conducted in the U.S. and Mexico in June.
The Novavax vaccine can also be stored in standard refrigerators, making it easier to distribute.
The EU previously announced that it had completed “exploratory talks” back in December 2020 on similar terms of ordering up to 200 million doses. But that deal was held up over delivery scheduling and supply chain issues. Furthermore, the Novavax vaccine has yet to clear the final hurdle of approval with either European or U.S. regulators.
Where is the vaccine going?
Last year, the U.S. gave Novavax a $1.6 billion grant in exchange for 100 million doses of the shot. But a series of production delays, plus ample supplies of the mRNA vaccines supplied by Pfizer and Moderna, as well as Johnson & Johnson’s adenovirus-based shot, ultimately put the vaccine candidate in low demand. The United Kingdom also ordered 60 million doses of the vaccine in March, which never arrived.
The EU wants 70% of its citizens to be fully vaccinated by the end of the summer, well before Novavax vaccines would be available in Europe. If the Novavax vaccine were to get approved in time, the member states would have the option to take the vaccine themselves, redirect them to other European countries, or ship them abroad to more vaccine-needy countries.
Novavax seems resigned to the fate that its COVID vaccine will find a home in those countries trailing in their vaccination efforts. CEO Stanley C. Erck told the Associated Press in June, following a batch of impressive clinical trial results, that “many of our first doses will go to…low- and middle-income countries, and that was the goal to begin with.”
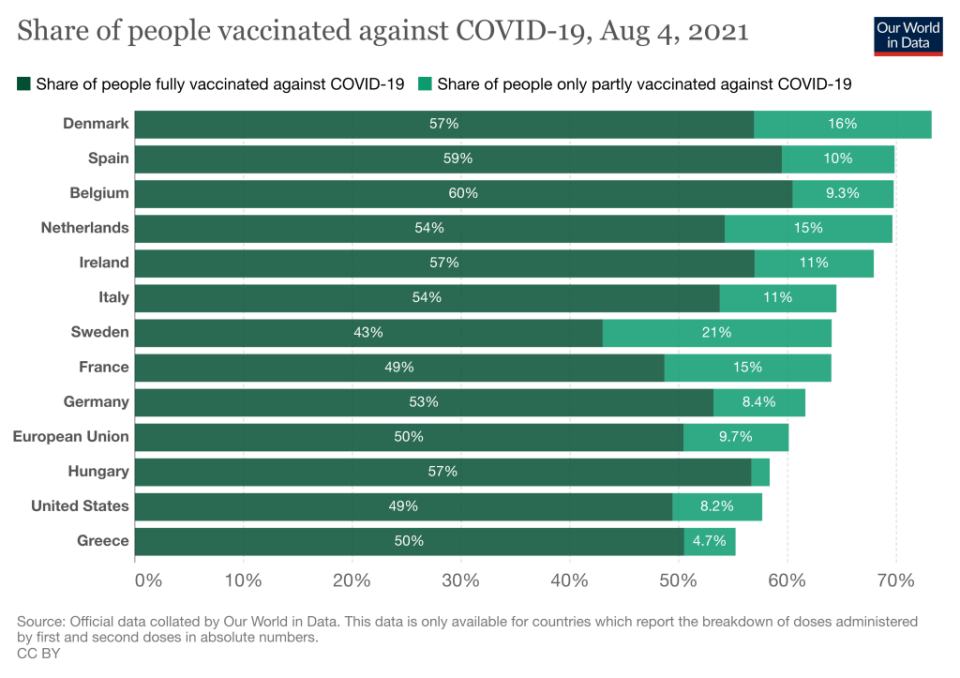
After a slow start, Europe has been rapidly vaccinating adults. But there are still big gaps between member states even as the Delta variant rips through the unvaccinated population. The EU vaccination rate, counting first and second jabs, hovers around 60%. It recently surpassed the United States in that much-watched measure.
This story was originally featured on Fortune.com

