More blood pressure medication has been recalled for having too much of an impurity
The latest blood pressure medication recall comes from Aurobindo Pharma, which yanked two lots of Quinapril and Hydrochlorothiazide tablets for having too much of the nitrosamine impurity N-Nitroso-Quinapril.
Here’s what you need to know.
Exactly what blood pressure drugs are recalled?
The recall concerns lot Nos. QE2021005-A and QE2021010-A of Quinapril and Hydrochlorothiazide Tablets USP 20mg / 12.5mg in 90-count bottles from Aurobindo Pharma with an expiration date of January 2023.
READ MORE: Pfizer recalls brand name, generic blood pressure medications for carcinogen content
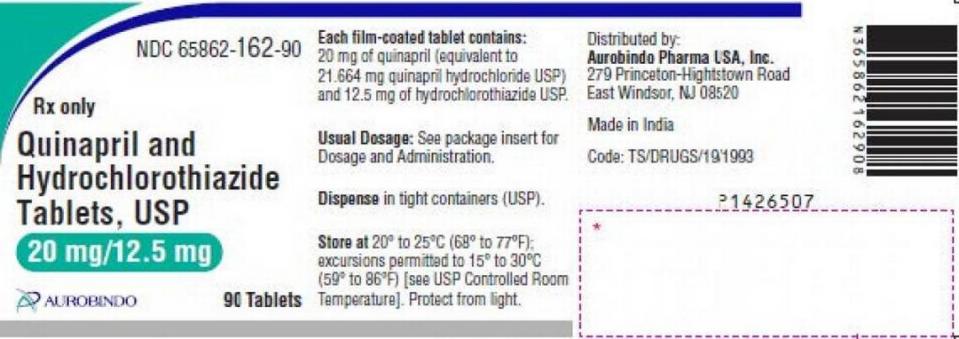
The company-written, FDA-posted recall notice describes the tablets as: ““Pink colored, scored, round shaped, biconvex, film-coated tablets, debossed with ‘D’ on the scored side and ‘19’ on the other side.”
The danger
While the recall notice’s risk statement opens with, “Nitrosamines are common in water and foods, including cured and grilled meats, dairy products and vegetables. Everyone is exposed to some level of nitrosamines,” it continues with: “These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time.”
READ MORE: Blood pressure and heart medications recalled. Pills were put in the wrong bottles
What you should do now
You’re not in immediate danger. But reach out to your doctor or pharmacist to discuss whether to keep taking this medication until a replacement treatment is decided.
READ MORE: About 37 million bottles of Pine-Sol recalled. They might have a bacterial problem
If you have questions about returning the recalled drugs, call Qualanex, which is handling the recall for Aurobindo, at 888-504-2014, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
If these or any drugs cause a problem, after notifying a medical professional let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Then notify the manufacturer, Aurobindo in this case, at 866-850-2876 (Option 2), 24 hours per day, 7 days per week or by emailing pvg@aurobindousa.com.

