Delaying Alzheimer's: Norton begins administering new drug to slow dementia progression
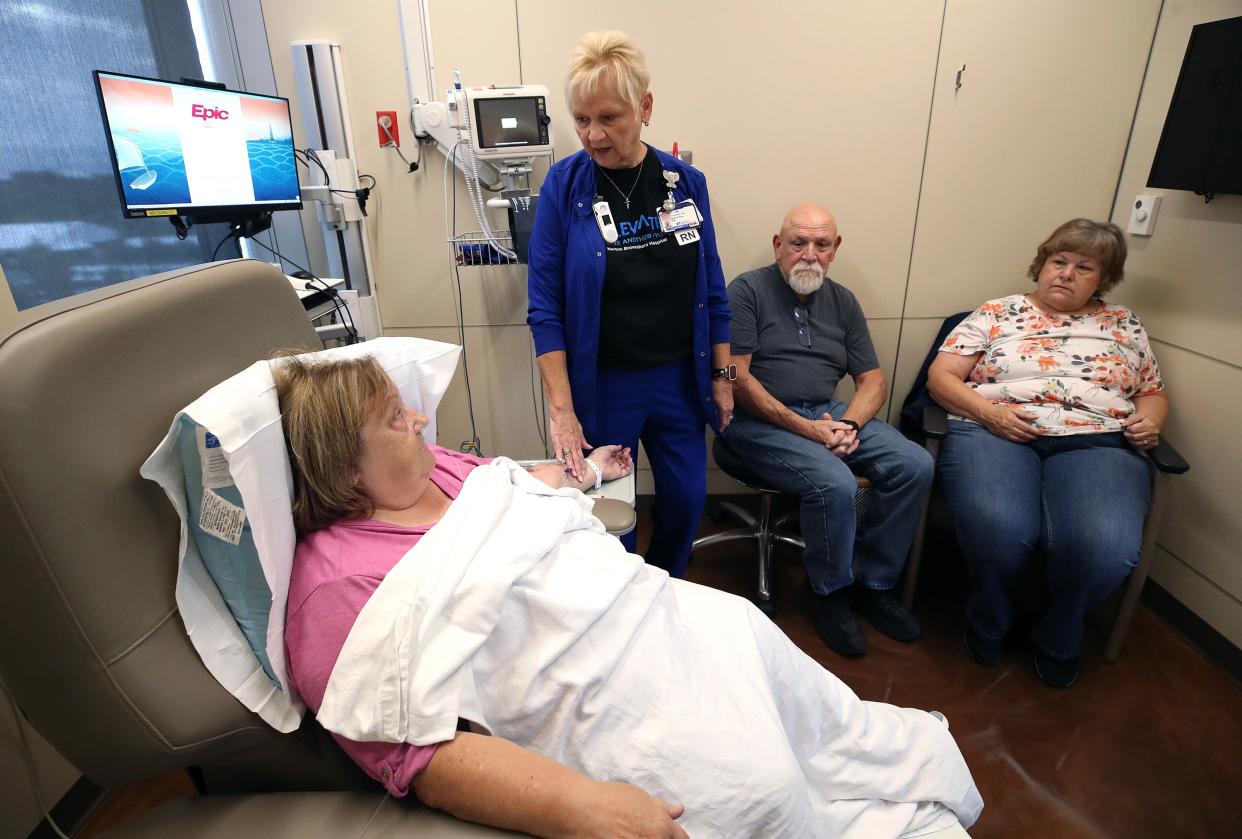
Every two weeks, Pam Jeffries settles into an armchair and waits for an infusion of a groundbreaking treatment for her memory loss.
And each time she does it, she wonders: Why?
Even if the drug slows her Alzheimer's disease as well as it did in the 18-month clinical trial, her husband Rick Jeffries will still need to have the same conversation with her week after week. In late August, Pam became the first person at the Norton Neuroscience Institute to have an infusion of Lecanemab. The program is the first of its kind in the region.
The new drug, which was approved by the FDA earlier this year, won’t fix Alzheimer's or dementia, but it may buy Pam more time with her memories. Studies show that Lecanemab can slow the disease's progression by 30%.
Since Pam, 73, was first diagnosed in 2021, her short-term memory has depleted so much that she can’t remember she has a mild cognitive impairment. She still speaks animatedly about her childhood and raising her daughters as a young, single mother in the 1970s, but she can’t recall who visited with her yesterday or even what she might have fixed for lunch an hour before.
So every 14 days, from now until likely the end of her life, Rick will kindly explain to his wife of 37 years she has a memory issue. Ideally, she’ll tell him that she trusts him to do what’s best, just like she did the first two times the IV went into her arm, so she could take Lecanemab.
Rick knows the infusion won’t make that conversation any easier.
But it may help Pam remember she trusts him.
Even if it’s just for a little bit longer.
The most important step toward a cure for Alzheimer's and dementia?
There is no cure for Alzheimer's and dementia, and for many families, the diagnosis begins a long, grief-filled decline to the end. But for the Jeffries and other families in the early stages of dementia, Lecanemab offers some hope.
Dr. Greg Cooper, the chief of adult neurology and director of the Memory Center at Norton Neuroscience Institute, whose team was the first in this region to administer Lecanemab, is candid about the treatment's limitations.
Somewhere between one-sixth and a third of Alzheimer's patients will be candidates for the infusions, and it will only help those in mild stages. The drug doesn’t stop memory loss, but when it removes an abnormal protein that appears in Alzheimer’s patients’ brains ― it can slow the progression of the disease.
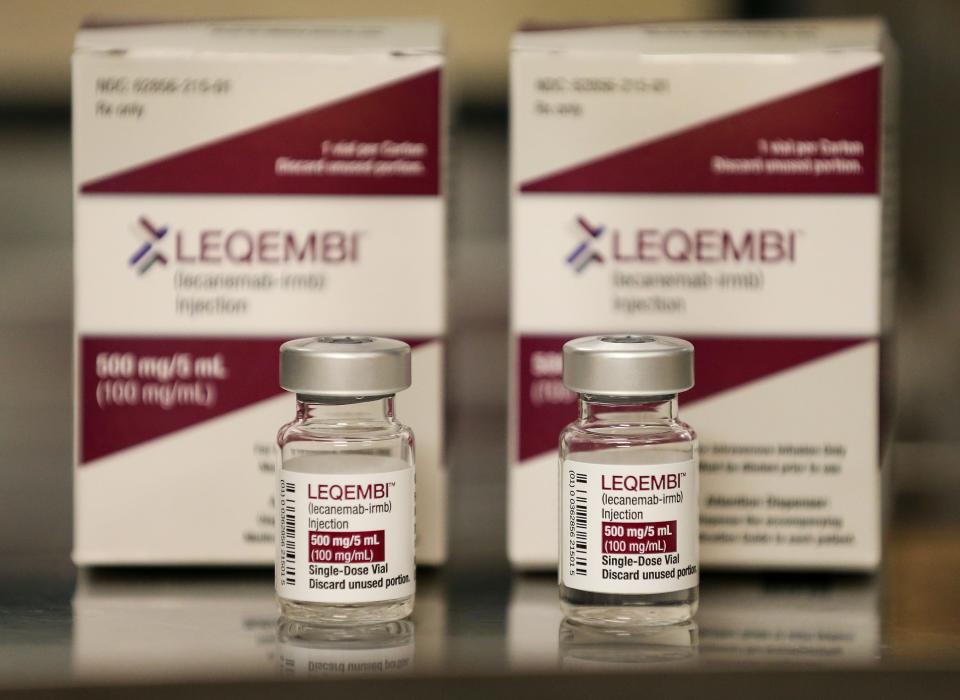
At first glance, it is difficult to tell just how much the illness has already taken from the Jeffries. The couple had planned to spend their 70s traveling, taking cruises, and socializing with friends, but now, it’s hard to motivate Pam to leave the house. She struggles to sleep at night, so she's sluggish during the day. Every so often, Rick will find his wife in her old office flipping through the pages of the memoirs she’d started working on before her illness. Now, she doesn’t write anymore.
Her daughter, Lisa Scott, was diagnosed with multiple sclerosis 18 years ago, and up until Pam’s memory began fading, she was Lisa’s rock through every treatment and surgery. That's changed a lot in the past year and a half. During a recent health scare, Pam could tell something was wrong because her daughter was using a walker, but her mind wouldn’t let her remember anything about what had happened.
“Why do you have a walker,” Pam would ask.
Her instincts to care and worry about her family are still very much there.
“Does your knee hurt?” Pam would say, just moments later.
After a while, it was easier for Lisa to just agree. The alternative was explaining the situation again and again.
“You just keep repeating and keep repeating, and it’s hard,” Lisa explained. “You have to remember that she can't help it. It's easy to get frustrated. You have to take a deep breath yourself, and realize she can't help it.”
On paper that 30% slow-down may not seem like a lot, but it can buy a family like the Jeffries more quality time and happy moments.
In terms of medical breakthroughs, this isn’t where science needs to be, Cooper explained, but up until this point, doctors have only been able to treat Alzheimer's symptoms.

“When the historians look back on this, I think we will see this is one of the most important steps toward the eventual cure of Alzheimer's disease,” Cooper said. “This is the first treatment that addresses what we believe is the underlying cause and really bends the curve and slows the progression of the disease.”
The Alzheimer’s Association estimates more than 6.7 million Americans are living with the illness.
As with any drug, Lecanemab has side effects such as headaches and allergic reactions to consider. The hour-long infusions every two weeks are arguably a big commitment for caregivers. Even with the frustrations that may come with forgetfulness, helping patients in that mild range of cognitive impairment is incredibly meaningful.
Preservation is powerful, Cooper says, even if it’s not necessarily long-term. When he first began speaking with patients and families about the potential, naturally, he expected some wouldn't be interested.
That hasn't been the case at all, though. Every family navigating mild cognitive impairment that he'd met with as of early September had wanted to give it a try.
'The one thing that always scared us'
The Jeffries were all too familiar with Alzheimer's when Pam first started forgetting things. Pam’s parents both had memory loss later in life, and Rick’s mother did, too.
“The last thing we wanted was to run into the issue where we don't have our minds,” Rick remembered. “That’s the one thing that always scared us.”
One afternoon when Rick’s mom was 86 years old, she took a long, hard look at him. Then she paused, confused. She clearly recognized her son, but her mind couldn’t find his name.
“Get over here big boy,” she finally called out from her hospital bed.
“I don't care what you call me,” he remembers telling her. “As long as you can recognize me, that's fine.”
Pam had already survived breast cancer and uterine cancer when forgetfulness set in. At first, when it was small things like losing keys and repeating herself, Rick didn't think anything of it. At 71 years old, Pam was so much younger than their parents had been. The Jeffries thought they'd have years before they faced something like this, and until they heard about Lecanemab, there was very little they could do to help it.
After Pam went for the first infusion, friends, family and even nurses asked Rick if he'd noticed a change in her.
It’s easy to wish and wonder, but this isn't about making the memory loss go away. The best Rick and Lisa can hope for is that they don't notice it getting worse.
“That does make this difficult because everybody wants to see an improvement,” Cooper said. “How do you measure lack of change? That's very hard.”
On the morning of Pam’s second infusion, a small crowd of medical professionals swirled outside of her room. She won’t remember all the faces and people she meets, of course, but as the first patient in Kentucky to have an infusion, she became a celebrity of sorts at the Norton Neuroscience Institute.
Everyone wants to see that she’s comfortable. Everyone wants these infusions to work.
In a way, everyone is latching onto that hope.
Until Lecanemab, early detection and diagnosis of Alzheimer’s really only offered families more time to plan for the worst, but that's not necessarily the case now. Pam’s age and mild status make her an ideal patient for treatment. If her daughters and husband had brushed off her repetition and forgetfulness as old age, Pam could have missed the window.
That window is what the Jeffries hope other families take away from Pam's story. When Pam beat cancer, she volunteered her time and resources to help patients. She wanted to lift up and comfort others.
Pam is the first Alzheimer’s patient in Kentucky to get a shot at keeping her memory just a little bit longer.
And while she’ll never truly remember this medical milestone, Rick and Lisa are confident she’d want to help others have that chance, too.
Reach Courier Journal features columnist Maggie Menderski at mmenderski@courier-journal.com.
Learn more about Lecanemab
To find out more information about Lecanemab, visit nortonhealthcare.com/lecanemab or call 502-446-4664.
This article originally appeared on Louisville Courier Journal: lecanemab-fda-delaying-alzheimers-norton-new-drug-slow-dementia

