Coronavirus update: Moderna vaccine 94.5% effective, US sets new record for daily cases
Moderna (MRNA) released a preview of its COVID-19 vaccine data Monday, saying its vaccine candidate is 94.5% effective and that it has an even longer shelf life in the refrigerator than previously estimated.
Both put the once-small biotech firm ahead of pharmaceutical giant Pfizer (PFE), whose candidate with BioNTech (BNTX) has proven to be more than 90% effective, according to the companies last week, but requires extra care in the form of ultra-cold storage to survive for a longer period of time.
Moderna said its vaccine can now survive for 30 days in regular refrigerator temperatures, and up to 6 months in -20 degrees Celsius. This could solve the limitations surrounding who can be shipped the vaccine, such as rural residents, that Pfizer faces.
Read more: Coronavirus vaccine: Cold storage remains hurdle for Pfizer vaccine distribution
U.S. Health and Human Services Sec. Alex Azar said in a briefing Monday that Moderna’s temperature for vaccine stability, as well as the doses per package, makes it “more amenable” to delivery needs for community pharmacies.
“Since early January, we have chased this virus with the intent to protect as many people around the world as possible. All along, we have known that each day matters. This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease,” said CEO Stéphane Bancel in a statement.
Unlike Pfizer, Moderna received federal funding from the National Institutes of Health to develop the vaccine, giving President Donald Trump and his administration a win amid the election loss.
Another Vaccine just announced. This time by Moderna, 95% effective. For those great “historians”, please remember that these great discoveries, which will end the China Plague, all took place on my watch!
— Donald J. Trump (@realDonaldTrump) November 16, 2020
“The Moderna/NIH vaccine candidate is now the second vaccine to show the potential for very high efficacy in Phase 3 trials. Operation Warp Speed has provided about $2 billion in funding and operational support for development, manufacturing, and eventual potential delivery of the Moderna/NIH vaccine,” Azar said.
Moderna said it plans to file for an emergency use authorization with the U.S. Food and Drug Administration “in the coming weeks.” And Bancel told Yahoo Finance the next steps, including figuring out the target populations for the vaccine, as well as working toward vaccines for children by the end of 2021.
Operation Warp Speed (OWS) officials said the micro-planning of distribution, including which populations and regions will be prioritized, is still in the works. OWS has contracted with McKesson for distribution of Moderna’s and other candidates, and pharmacies including CVS (CVS), Walgreens (WBA), Rite Aid (RAD), as well as retailers like Walmart (WMT) and Kroger (KR) are set to administer the vaccines.
But the pharmacies are still going to provide a limited amount of the total vaccinations. At least 32% will flow through these outlets, according a note from Nephron Research Monday.
While both the frontrunners in the U.S. are mRNA technology-based, the remaining candidates of Operation Warp Speed use different technologies, which prevents OWS from getting more involved on the production side.
Bancel said technologies for mRNA production are new and not been manufactured on a large-scale before. Swiss company Lonza, which partnered with Moderna, had to retool entire rooms to be able to accommodate the needs of the biotech.
Moderna anticipates producing 20 million vaccines by the end of the year with 40 million per month after. The exact numbers are hard to predict because of how vaccines are made, Azar explained.
“This is not like making a pill where there is a mathematical certainty,” he said, adding there is a “scientific uncertainty” for the yield of each batch being produced.
Record cases
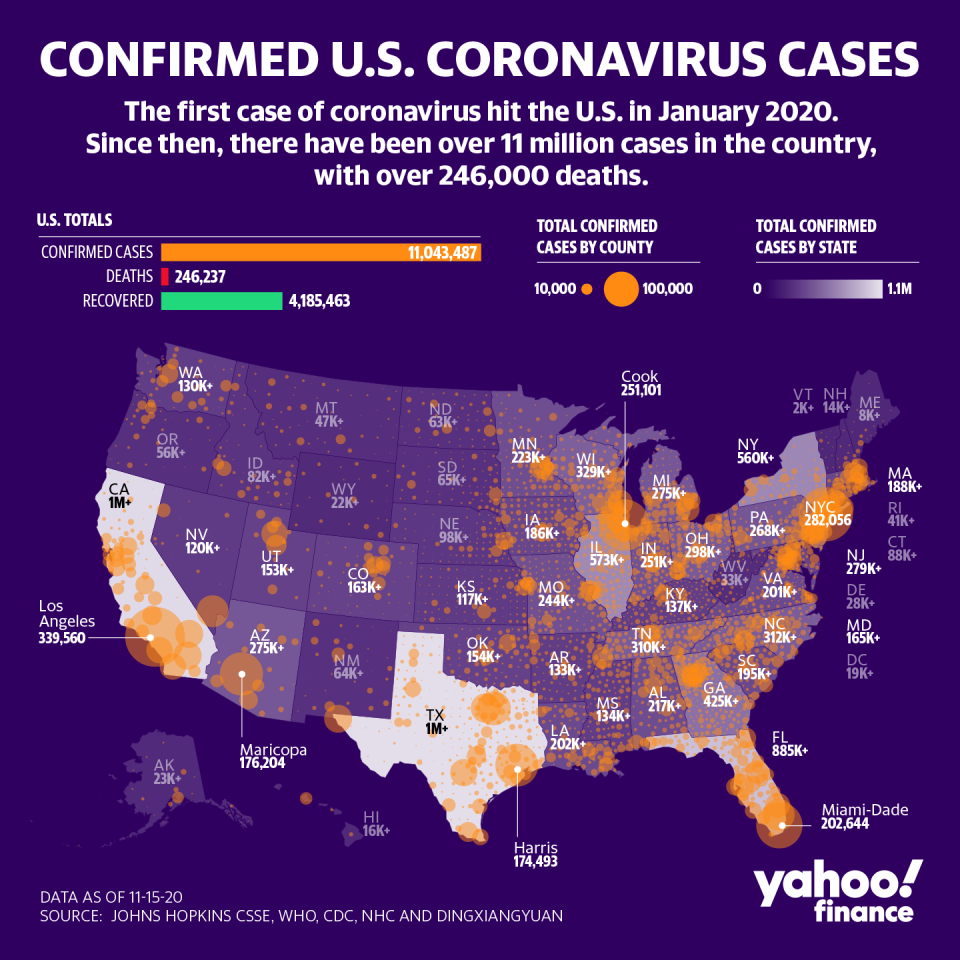
The news is welcome after the weekend brought grim news as the US set a new record in daily coronavirus cases with more than 180,000 reported Friday.
The sharp rise in cases pushed the total case count to more than 11 million Monday, up from 10.5 million prior to the weekend. Its why all government officials re-iterated the need to wash hands and wear masks as well as maintain social distancing during the media call Monday morning.
Following those rules will “bridge us to that bright future,” Azar said. “It is absolutely unprecedented to have two vaccines with highly promising data in less than a year” after the emergence of a global emergency.
Especially since, while the news is positive, much remains unknown. That includes how long the protection lasts.
“We do not know at this point what the durability and protection will be,” said National Insitute of Allergy and Infectious Diseases director Dr. Anthony Fauci.
He added that the most optimistic scenario is for the general public to begin getting vaccinated would be April.
More from Anjalee:
Fauci: Vaccines will only prevent symptoms, not block the virus
A Biden win could lead to a mask mandate, more testing: Expert
Trump admin rules for health insurer price transparency has potential to curb costs
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.

