Another blood pressure medicine recall for bottles having tablets of the wrong strength
For the second time this month, there’s a recall of a blood pressure medication because the tablets inside the bottle don’t match the label outside the bottle.
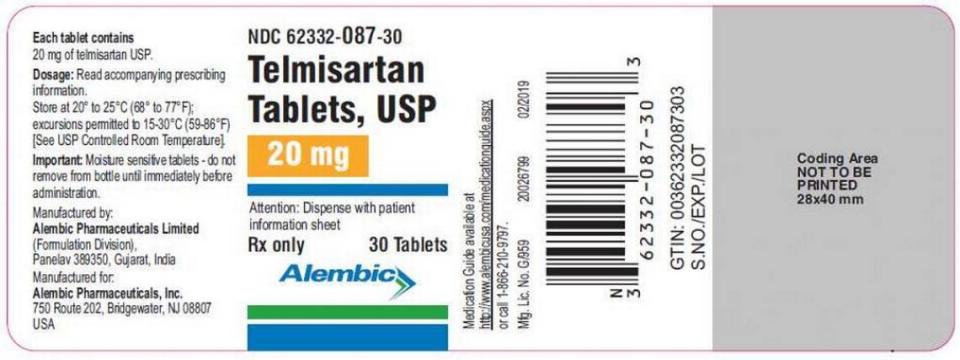
Thursday’s announcement concerns one lot of Alembic Pharmaceuticals’ 30-count bottles of 20 mg Telmisartan Tablets. The company received a complaint that such a bottle of the hypertension drug contained 30 tablets, but 40 mg strength tablets instead of 20 mg, as the label states.
As for the problem that could create, the Alembic-written, FDA-posted recall notice says, “Patients who could be on a doubled dose of telmisartan for a prolonged period of time, could experience low blood pressure, worsening of kidney function, or an elevation of potassium which can be life-threatening.”
Drug used to bring blood pressure back up after surgery is recalled. It may not be sterile.
Clinical trials in Kendall for addiction and diabetes drugs were fraudulent, feds say
This involves lot No. 1905005661 with a March 2022 expiration date and NDC No. 62332-087-30.
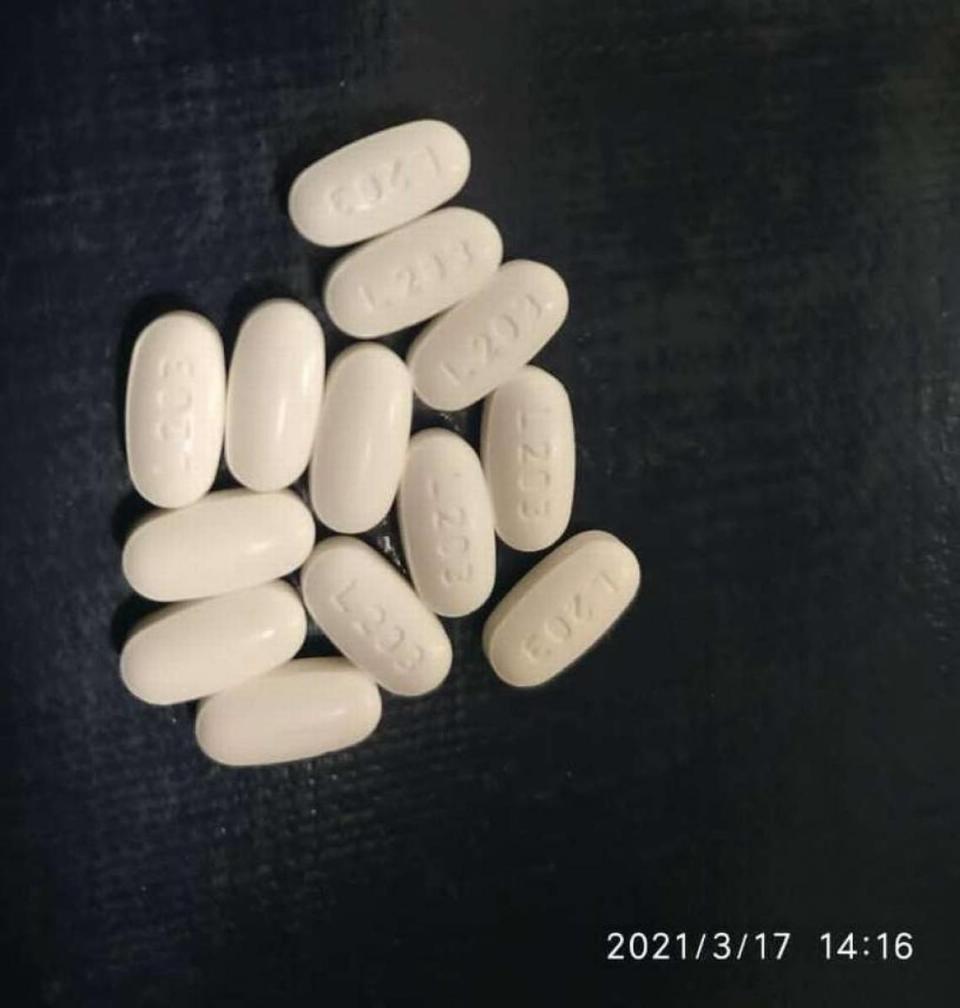
Also, the 20 mg tablets are round with “L202” on one side. The 40 mg tablets are thicker, oval-shaped and have “L203” on one side.
If this sounds familiar, just two weeks ago, Bryant Ranch Prepack recalled four lots of Spironolactone after a similar packaging error might have mixed up two strengths of that drug.
Consumers should talk to their pharmacist or medical professional about a replacement treatment. Then, return the medication to the store of purchase.
If this or any other drug causes a medical problem, after notifying a medical professional, let the Food and Drug Administration know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088.
Questions about the recall should be directed to Alembic, either by emailing david.cobb@alembicusa.com or calling 908-552-5839, Monday through Friday, 9 a.m. to 5 p.m. ET.
Miami plastic surgeon disappeared as his patient bled into unconsciousness, state says
A South Florida doctor almost killed a baby during circumcision, the state says

