37 lots of a mouthwash have been recalled for a possible bacterial contamination
Sunstar Americas recalled 37 lots of GUM Paroex Chlorhexidine Gluconate Oral Rinse after finding the mouthwash might be a Burkholderia lata contamination problem.
As explained in the Sunstar-written, FDA-posted recall notice, that’s a bacteria that can cause “oral and, potentially, systemic infections requiring antibacterial therapy. In the most at-risk populations, the use of the defective product may result in life-threatening infections, such as pneumonia and bacteremia.”

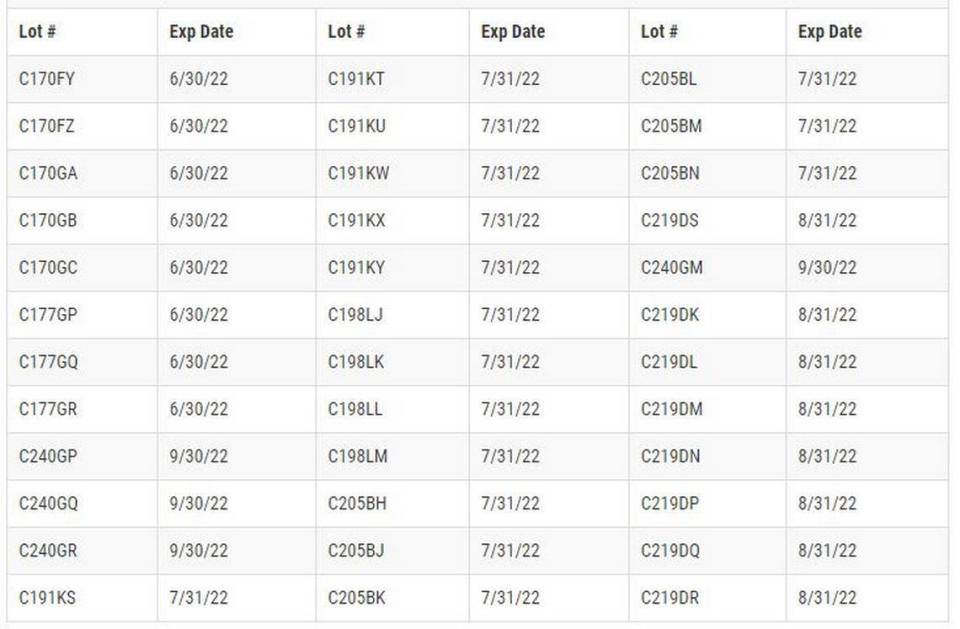
This prescription mouthwash treats gingivitis. What’s recalled comes in 4-ounce bottles, lot No. C191KR with an expiration date of 7/31/22; and 16-ounce bottles, 36 lots with lot Nos. between C170FY, expiration 6/30/22, and C219DR, expiration 8/31/22.
If you suffer a medical problem from this or any other drug, you should first contact a medical professional. Then, report the problem to the FDA’s MedWatch Adverse Event Reporting program either via the FDA website or by a form obtained at 800-332-1088.
Customers with questions about this recall can email us.pcr@us.sunstar.com or call 800-528-8537, Monday through Friday, 9 a.m. to 6 p.m., Eastern time.

Miami-Dade company recalls stone crab sauce distributed as stone crab season begins
There’s a problem with some of Trader Joe’s fish. Over 4,400 pounds have been recalled

