In abortion pill hearing, Supreme Court sounds skeptical of challenge to mifepristone access
A high-stakes hearing played out before the U.S. Supreme Court on Tuesday in a case that could reshape abortion access nationwide.
The justices considered a challenge to the Food and Drug Administration’s regulation of mifepristone, the first pill taken in a two-drug regimen for a medication abortion, which is the most common method of abortion in the country.
It is the first major reproductive rights case before the high court since Roe v. Wade was overturned in 2022. A decision is expected by the end of June.
Latest Developments
Mar 26, 2:11 PM
Most justices sound skeptical of restricting mifepristone
With the hearing wrapped, after about two hours of arguments, the Supreme Court appeared highly skeptical of the challenge to the mifepristone regulations brought by a group of anti-abortion doctors -- suggesting recent steps to ease access to the medication used by millions of American women may be allowed to stand.
At the heart of the case are steps taken by the FDA in 2016 and 2021 to roll back safety measures around the pill, which was first approved for use in 2000.
The plaintiff doctors -- who do not prescribe mifepristone, use mifepristone or otherwise perform abortions -- claim that wider availability of the drug was authorized improperly and has adversely impacted them, forcing them to care for women in emergency rooms suffering complications from the pill, often in violation of their conscience.
While conservative-leaning Justices Samuel Alito and Clarence Thomas were the most sympathetic to the legal challenges, for the most part, the court on Tuesday steered clear of openly second-guessing FDA’s scientific analysis of mifepristone.
A majority of the justices, across the ideological spectrum, expressed doubt during the arguments that the doctors had sufficiently demonstrated legal standing that they had been directly harmed by the FDA’s mifepristone rule changes.
“It makes sense for individual doctors to seek a [conscience] exemption but they already have that,” said liberal-leaning Justice Ketanji Brown Jackson. “What they are asking for here is -- in order to prevent them from ever having to do these kinds of procedures -- that everyone else should be prevented from getting access to that medication. How is that not overbroad?”
That view was echoed by conservative-leaning Justice Neil Gorsuch.
Erin Hawley, the attorney for the doctors, was repeatedly pressed to provide specific examples or testimony from a physician who had been forced to violate his or her conscience in treating a mifepristone patient but she could not do so.
Solicitor General Elizabeth Prelogar, defending the FDA on behalf of the Biden administration, warned of "profound harm" for women and for drug companies working with the FDA should the lower court's restrictions on mifepristone remain.
-ABC News' Lalee Ibssa and Devin Dwyer
Mar 26, 12:29 PM
Government gives brief rebuttal as oral arguments come to a close
Solicitor General Elizabeth Prelogar gave a brief rebuttal on behalf of the federal government, summarizing their view that respondents do not have standing and that their sought remedy of nationwide relief is not warranted.
"They have said they fear there will be emergency room doctors somewhere, someday who might be presented with some woman suffering an incredibly rare complication and the doctor may have to provide treatment, notwithstanding the conscience protections," she said.
"We don't think that harm has materialized -- but what the [lower] court did to guard against that very remote risk was issue sweeping nationwide relief that restricts access to mifepristone for every single woman in this country and that causes profound harm," she added. "It harms the agency, which had the courts come in and displace the agency's scientific judgments. It harms the pharmaceutical agency that is sounding alarm bells that this would destabilize the system for approving and regulating drugs. And it harms women who need access to medical abortions under conditions the FDA determined were safe and effective."
Mar 26, 11:54 AM
Justices ask how often abortion complication requires surgery
Erin Hawley, the attorney for Alliance Defending Freedom representing the plaintiffs, said they are "harmed" without an injunction of mifepristone because it may require them to treat people experiencing abortion complications and "take an unborn life."
Justice Ketanji Brown Jackson asked Hawley asked how often would it be that the plaintiffs have to complete the procedure "in the way that you are describing?"
Hawley said plaintiffs have treated people experiencing abortion complications "dozens of times" and even said treatment may require "scraping out a uterus". However, she did not provide specific numbers.
Mar 26, 12:34 PM
Kagan presses attorney for anti-abortion group on which specific doctor is injured
Justice Elena Kagan, continuing the court's interest in the plaintiff's standing to bring this case, pressed attorney Erin Hawley on her "conscience harm" argument.
"You need a person. You need a person to be able to come in and meet the court's regular standing requirements," Kagan. "So, who's your person?"
Hawley pointed to one doctor, Dr. Christina Francis, whose partner had to perform a dilation and curettage (or a D&C) due to a life-threatening emergency for a woman who had taken abortion medication. Kagan asked if the doctor stated her objection at that time, which Hawley said they did not.
"The way people with conscience objections do this is they make those objections known," Kagan said. "That may be harder, that may be easier in a particular context but most hospitals have mechanisms in place, routines in place to ensure the doctors who are allowed to do this in advance and are allowed to it at the moment."
Mar 26, 11:25 AM
Justices wonder why nationwide injunction of mifepristone is needed
Justice Ketanji Brown Jackson asked Erin Hawley, the attorney for Alliance Defending Freedom -- the group representing the plaintiffs -- if a nationwide reversal of the FDA's approval of mifepristone is needed and if the court can simply rule that doctors do not need to prescribe the pill if they have conscience objections.
"Do we have to entertain your argument that no one in the world can have this drug in order to protect your client?" the justice asked.
Hawley argued that given the "emergency nature," it is "impracticable" to not have a broader ban or block on access to the pill.
Justice Neil Gorsuch echoed Jackson's question and asked if it is enough for the court to rule on behalf of the plaintiffs not being required to prescribe the pill and why the court has to consider a universal injunction.
Mar 26, 11:59 AM
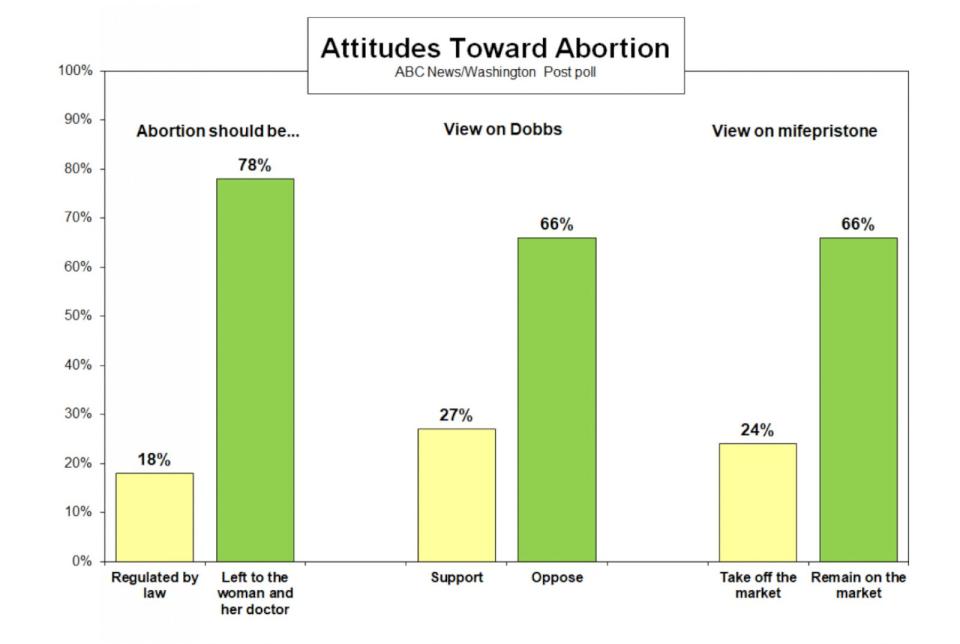
What Americans think of the abortion pill
Two-thirds of Americans (66%) said mifepristone should remain on the market in an ABC News/Washington Post poll conducted last year.
Plus, 72% of Americans said at the time that access to the drug should remain the same.
Overall, 78% of Americans said the decision whether to have an abortion should be left to a woman and her doctor rather than regulated by law. That view was shared by 58% of Republicans.
The poll (conducted 10 months after the fall of Roe) also found half of Americans thought the Supreme Court justices base their rulings mainly on their personal political opinions, not on the law. Before the abortion ruling, the public was divided 46%-45% on whether the justices' rulings were based mainly on the law or on their own political preferences.
Mar 26, 3:58 PM
Anti-abortion group begins presentation to court
Attorney Erin Hawley, representing the anti-abortion Alliance for Hippocratic Medicine that is challenging mifepristone, has begun her presentation to the justices.
Hawley is echoing the alliance's attacks on the drug's use and regulation by the FDA, contending that it creates harm to women and burdens doctors who must then care for such patients who use mifepristone.
Hawley also invoked an "intolerable" choice that is created by the widespread use of mifepristone -- if an anti-abortion doctor must consider treating abortion patients in an emergency situation or not.
-ABC News' Adam Carlson
Mar 26, 2:19 PM
Attorney notes some studies in initial ruling were retracted
Jessica Ellsworth, the attorney for Danco Labs, was asked by Justice Ketanji Brown Jackson if she was concerned about the prospect of judges parsing medical and scientific studies without specialized knowledge.
"I think we have significant concerns about that," Ellsworth said, noting the pharmaceutical industry submitted briefs to the court expressing that worry.
She went on to state that U.S. District Judge Matthew Kacsmaryk, who initially ruled to suspend mifepristone's approval, relied on studies that "were not in the administrative record" and never would have been.
"They have since been retracted for lack of scientific rigor and misleading presentations of data," she said.
Sage Publishing said it issued the retractions from the journal Health Services Research and Managerial Epidemiology because of methodology issues and conflicts of interest, ABC News previously reported.
Mar 26, 11:12 AM
Justices ask if FDA should have continued requirement of reporting mifepristone 'harms'
Justice Samuel Alito asked Jessica Ellsworth, the attorney representing Danco Laboratories, if she thinks the FDA should have continued requiring prescribers to report non-fatal complications, known as “adverse events" of mifepristone.
Ellsworth argued the FDA made that decision after 15 years of data that established mifepristone was safe. She also rejected Alito's question asking if the FDA was "infallible".
Justice Kentanji Brown Jackson asked if the defendants had concerns about judges "parsing medical and scientific studies" and Ellsworth said there were concerns.
Mar 26, 10:55 AM
Attorney for Danco Labs warns of broader consequences for drug approvals
Jessica Ellsworth, who is representing the manufacturer of Mifeprex (the brand name of mifepristone), similarly criticized the plaintiff's claim of injury and their interpretation of the law.
She said the respondent's view is "so inflexible it would upend not only Mifeprex but virtually every drug approval and [risk and mitigation] modification the FDA has made for decades."

