Pfizer (PFE) Gets Approval for New Eczema Drug in Europe
Pfizer PFE announced that the European Commission (“EC”) has approved its investigational once-daily JAK1 inhibitor, abrocitinib, for the treatment of moderate-to-severe atopic dermatitis (“AD”) in adults who are candidates for systemic therapy. The drug will be marketed by the tradename, Cibinqo.
Cibinqo will be available in two different doses — 100 mg and 200 mg — for the approved indication. Moreover, a 50mg dose of the drug will be available to treat AD patients specifically with moderate and severe renal impairment (kidney failure) or certain patients receiving treatment with inhibitors of cytochrome P450 (CYP) 2C19.
The approval for Cibinqo was expected as the European Medicines Agency's Committee for Medicinal Products for Human Use had recommended approving it in October. The approval for the drug was based on positive data from five phase III studies and a long-term extension study that evaluated abroticinib in more than 3,100 patients.
The top-line data from a late-stage study demonstrated that treatment with abroticinib resulted in statistically superior efficacy compared to Sanofi SNY and Regeneron’s REGN blockbuster AD drug, Dupixent (dupilumab), in each evaluated measure.
We note that dupilumab is a fully human monoclonal antibody developed by Regeneron and Sanofi as part of a global collaboration agreement. Sanofi and Regeneron plan to file a regulatory application seeking label expansion of Dupixent to include pediatric AD patients by the end of 2021.
Pfizer’s stock has risen 43.4% this year so far compared with an increase of 14.7% for the industry.
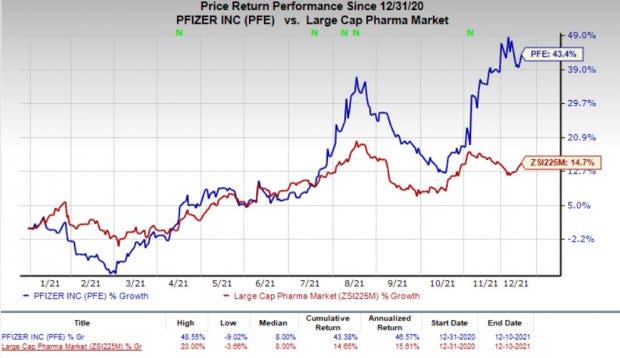
Image Source: Zacks Investment Research
Please note that the regulatory filing for the drug is already under priority review with the FDA. Also, the drug has already received marketing authorization in the U.K. and Japan this year under the trade name Cibinqo.
However, the FDA has extended review period of the new drug application (“NDA”) seeking approval for abrocitinib as a treatment for AD twice this year.
The FDA’s review of the final data from the post-marketing safety study in September showed that rheumatoid arthritis patients treated with both low as well as high doses of Pfizer’s another JAK inhibitor, Xeljanz, experienced a higher rate of serious heart-related events such as heart attack and stroke, cancer, blood clots, and death compared to those treated with TNF inhibitors. The FDA asked to add warnings about an increased risk of serious heart-related events, cancer, blood clots, and even death to Xeljanz’s label. This has led to the extensions of the abrocitinib NDA.
In a separate press release, Pfizer and its partner Sangamo Therapeutics SGMO announced follow-up data from a phase I/II study evaluating their gene therapy candidate, giroctocogene fitelparvovec, for treating moderately severe to severe hemophilia A.
Updated data from Pfizer and Sangamo’s study demonstrated no annualized bleeding rate in the one year post-infusion. PFE and SGMO are currently evaluating the candidate in a phase III study as a potential treatment for severe hemophilia A.
Pfizer Inc. Price
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank
Pfizer currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Sangamo Therapeutics, Inc. (SGMO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
