Problems from pain to cardiac flatline possible after a Pfizer company’s drug mix-up
One lot of 1% Lidocaine and one lot of 0.5% Bupivacaine have been recalled because some Lidocaine went out in bottles labeled as Bupivacaine and vice versa, a mistake that can cause patient death.
These anesthesia drugs, this mistake and this recall come from Hospira, a company owned by Pfizer. And, the Hospira-written, FDA-posted recall notice admits the mislabeling could bring “adverse events of moderate to high severity.”
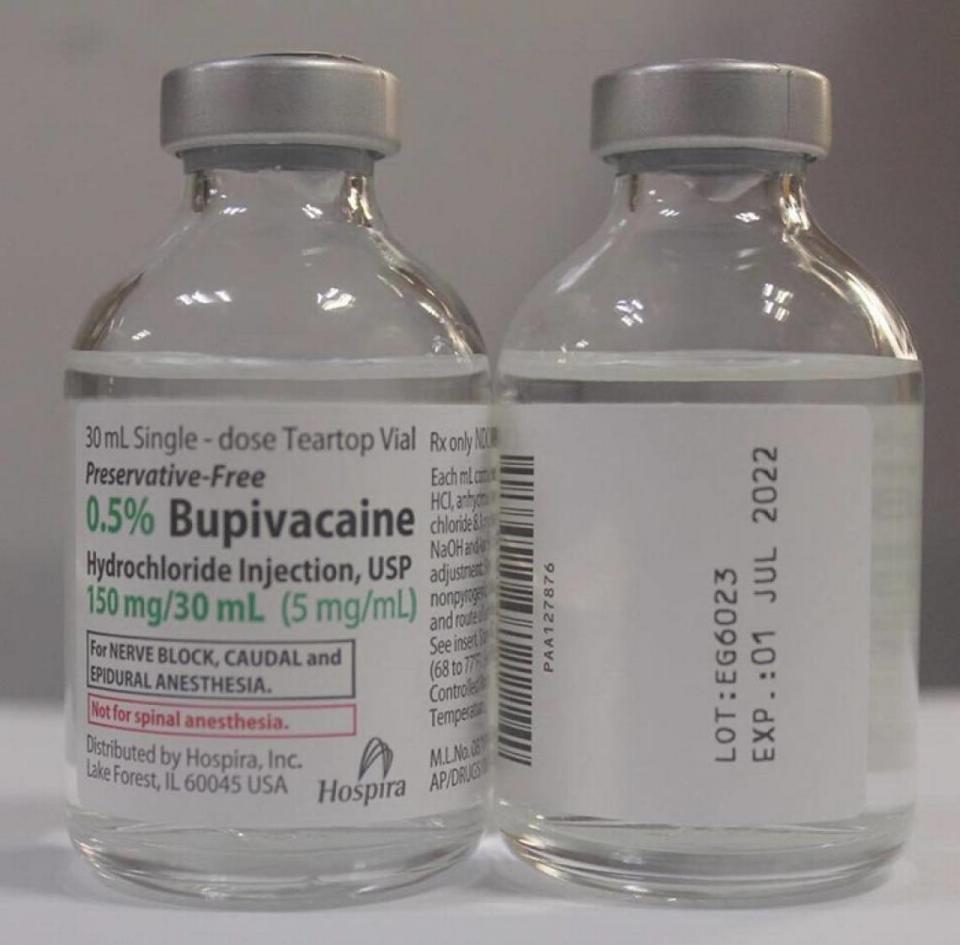
If you got 1% Lidocaine when 0.5% Bupivacaine is called for, Hospira’s alert says, you “may be underdosed” which means “inadequate pain management, and failure of surgical anesthesia.” Bupivacaine is used in dental and oral surgery, obstetrical and other procedures.
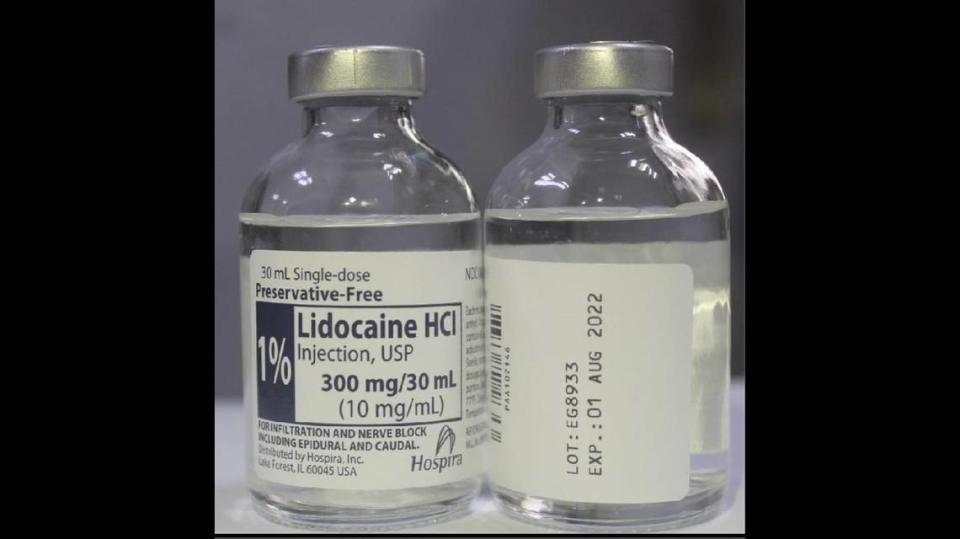
Here’s Hospira’s list of possible consequences of 0.5% bupivacaine instead of 1% lidocaine: “seizures; respiratory abnormalities including low oxygen and/or elevated carbon dioxide in the blood; too much acid in the body fluids, and temporary cessation of breathing; heart abnormalities such as heart contraction and/or relaxation issues; irregular heartbeat; slower than normal heart rate; abnormal heart rhythm in which the ventricles of the heart quiver instead of pumping normally; cardiac arrest; and cardiac flatline.”
This covers 0.5% Bupivacaine Hydrochloride Injection, USP, Single Dose Teartop Vial, lot No. EG6023, expiration 01 July 2022; and 1% Lidocaine HCl Injection, USP Single Dose Teartop Vial, lot No. EG8933, expiration 01 Aug. 2022. Each is in case packs of 2 x 25 vials.
For returning or handling of the drugs, call Stericycle at 800-805-3093, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
For medical questions about this recall, medical professionals should call Pfizer Medical Information at 800-438-1985, option No. 3, Monday through Friday, 9 a.m. to 5 p.m., Eastern time. To report an adverse event or file a complaint, medical professionals should call Pfizer Safety at the same number, option No. 1, 24 hours a day, seven days a week.
For patients, if this or any drug causes a medical problem, notify a medical professional, then let the Food and Drug Administration know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Only then should you consider calling the manufacturer.
A thyroid medicine’s third recall: It’s too weak and 43 people had ‘serious’ problems

