What to Know About Paxlovid, the Antiviral Drug That the Bidens Were Prescribed for COVID
- Oops!Something went wrong.Please try again later.
- Oops!Something went wrong.Please try again later.
Fabian Sommer/picture alliance via Getty Images
First Lady Jill Biden has tested positive for COVID-19.
"After testing negative for COVID-19 on Monday during her regular testing cadence, the First Lady began to develop cold-like symptoms late in the evening," read a Tuesday morning statement from her communications director, Elizabeth Alexander. "She tested negative again on a rapid antigen test, but a PCR test came back positive."
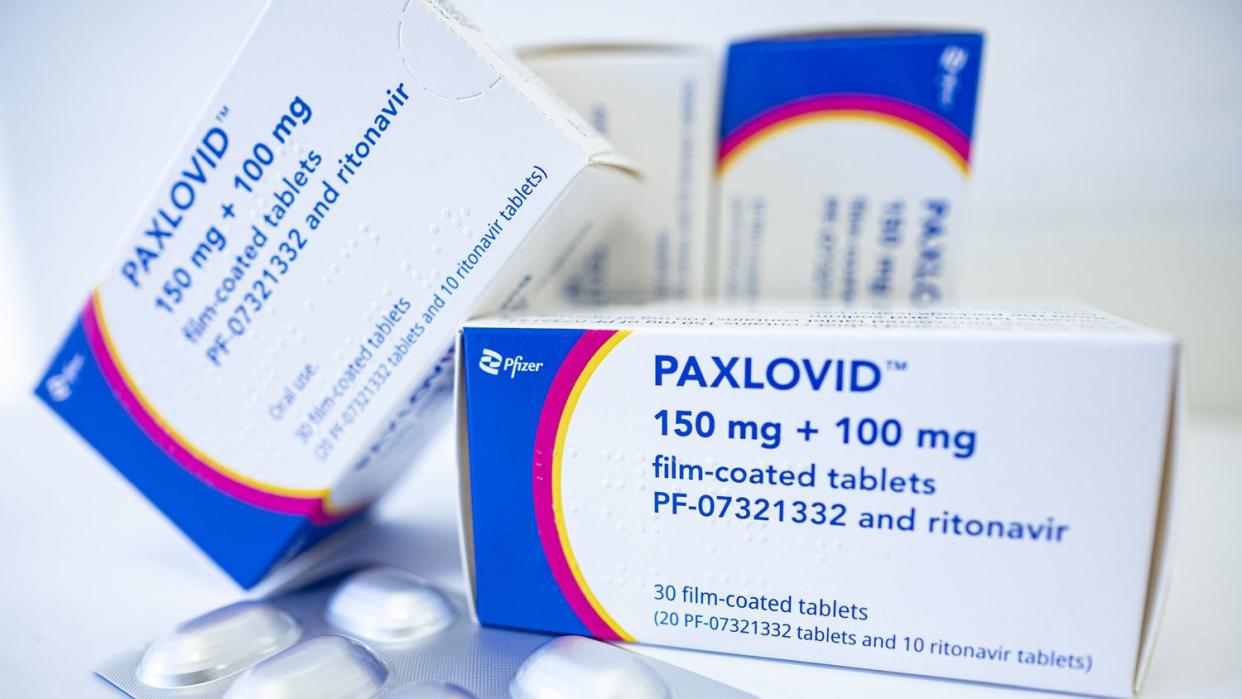
She has started taking Paxlovid, which President Joe Biden also took last month when he had COVID. Early use of the drug can help prevent serious illness.
RELATED: Dr. Fauci Takes Second Course of Paxlovid After Experiencing COVID-19 Rebound
Paxlovid is an oral antiviral medication that prevents high-risk patients from becoming too sick and requiring hospitalization. The drug is available with a prescription from a health care provider or from a pharmacist, and should be taken within five days of developing symptoms. The dosage is three pills, twice a day, for five days, or a total of 30 pills.
The tablet has shown the capacity to reduce the risk of hospitalization or death for high-risk adults with COVID-19 by 89%.
RELATED: 35 Companies to Produce Generic Version of COVID Pill that Helps Prevent Hospitalization and Death
Recently, the FDA authorized pharmacists across the United States to prescribe Pfizer's antiviral treatment pill to eligible patients.
Individuals who test positive for COVID and want to determine their eligibility to take Paxlovid can bring a list of their current medications and health records detailing kidney or liver problems to their pharmacist for review.
Patients with reduced kidney function may require a lower dose of Paxlovid, per the FDA.
The FDA first granted Paxlovid emergency use authorization in December 2021 for use in patients age 12 or older who are at a high risk of developing severe COVID-19 due to underlying medical conditions.
RELATED VIDEO: FDA Authorizes Pharmacists to Prescribe Pfizer's COVID-19 Antiviral Pill
In March, 35 manufacturers around the world signed on to produce the generic version of Paxlovid.
Production of the generic version will help citizens in 95 low and middle income counties have access to a more affordable version of the Pfizer COVID-19 treatment pill, says Medicines Patent Pool (MPP), a United Nations-backed nonprofit.
"We have established a comprehensive strategy in partnership with worldwide governments, international global health leaders and global manufacturers to help ensure access to our oral COVID-19 treatment for patients in need around the world," said Albert Bourla, Chairman and Chief Executive Officer of Pfizer per the news release.

