FDA Panel Votes to Move Forward with COVID Vaccines for Children as Young as 6 Months Old
- Oops!Something went wrong.Please try again later.
Getty Images
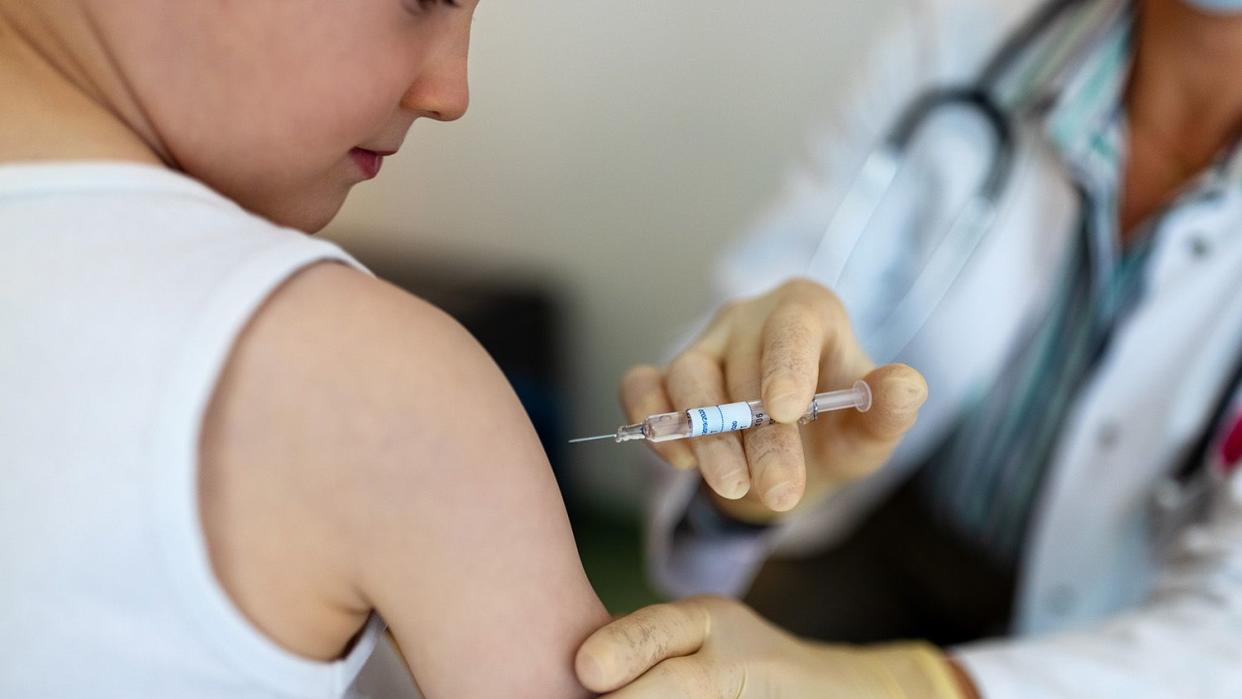
On Wednesday, a Food and Drug Administration panel voted unanimously to authorize use of the Moderna and Pfizer/BioNTech COVID-19 vaccines for children as young as six months old.
In the 21-0 vote, members of the FDA's Vaccines and Related Biological Products Advisory Committee all voted "yes" to the question, "Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine when administered as a 2-dose series (25 micrograms each dose) outweigh its risks for use in infants and children 6 months through 5 years of age?," CNN reported.
RELATED: Dr. Anthony Fauci Tests Positive for COVID and Is 'Experiencing Mild Symptoms'
Voters did the same, according to the outlet, in regard to the question of, "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine when administered as a 3-dose series (3 micrograms each dose) outweigh its risks for use in infants and children 6 months through 4 years of age?"
Now the decision goes to the FDA, who according to CNN, normally follow the committee's recommendations. Once they approve an emergency use authorization of the vaccines for this age group, an advisory panel at the Centers for Disease Control and Prevention is expected to meet on Friday or Saturday to vote on whether they will endorse the shots, NBC News reported.
CDC Director Dr. Rochelle Walensky needs to sign off before this age group can begin getting their shots, NBC News reported.
There are roughly 18 million children in that age group, according to the Associated Press,.
"This is a long-awaited vaccine," said one panel member, Dr. Jay Portnoy of Children's Hospital in Kansas City, Missouri, the AP reported. "There are so many parents who are absolutely desperate to get this vaccine and I think we owe it to them to give them a choice to have the vaccine if they want to."
Pfizer and BioNTech announced in December that a two-dose regimen did not elicit enough of an immune response in some children under 5, prompting their study of a third dose.
Getty Images
At the time, the companies reported that the two-dose vaccine was effective in children under age 2, similar to those in the 16-24 age bracket. However, children ages 2 through 5 generally did not have the same response.
In April, multiple sources told Politico that it could be June before the FDA authorizes a COVID vaccine for children under five years old. The outlet added that delays are being attributed to regulators who want to promote two vaccines at the same time instead of pushing one out before the other.
Vaccine advocates encouraged the administration to act sooner rather than later.
Colorado Gov. Jared Polis wrote a letter calling on the Biden administration to step up efforts to get authorization for vaccines for the country's youngest population.
RELATED: Around 1 in 5 Americans May Develop Long COVID, Large CDC Study Finds
"Hospitalization rates for children under 5 were the highest ever during the omicron surge. While children younger than 5 are less vulnerable to SARS-CoV-2 than adults, they can still experience severe and lasting outcomes," Polis said in the letter. "Delays and lack of urgency from the FDA and vaccine developers in authorizing a vaccine for children under 5 are concerning."
The Centers for Disease Control and Prevention first approved the Pfizer-BioNTech vaccine for emergency use in children ages 12 to 15 back in May 2021. The vaccine received the same approval for children ages 5 to 11 six months later in November.

