FDA Fully Approves New Drug That Can Slow Progression of Alzheimer's Disease
- Oops!Something went wrong.Please try again later.
Leqembi will be covered under Medicare, but some say the cost is too high — and side effects too risky
Getty
Groundbreaking new Alzheimer's drug Leqembi has been fully approved by the FDAThe FDA has fully approved a groundbreaking new drug for the treatment of Alzheimer’s Disease. But Leqembi carries a hefty price tag — and potential side effects that some deem too risky.
In January, the FDA granted an Accelerated Approval pathway for Leqembi, a new drug that appears to slow the progression of Alzheimer’s Disease. Since Leqembi classifies as a drug that “that [fills] an unmet medical need,” it qualified for the fast-tracked approval process.
But now it’s fully approved — which means it will now be covered under Medicare.
In June, the Centers for Medicare & Medicaid Services said it would pay for about 80% of Leqembi and other drugs in its class, pending full FDA approval.
Michael Robinson Chávez/The Washington Post via Getty Images
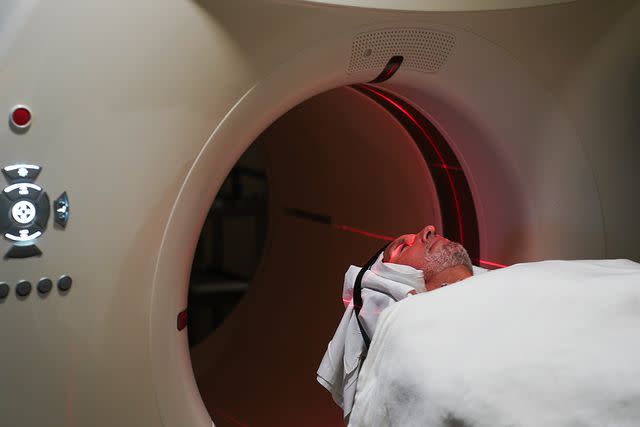
A patient receives a PET scan to determine if he's eligible for the new Alzheimer's drug, Leqembi.Without insurance, Leqembi will cost $26,500 a year. Under Medicare, its cost could come to around $5,000.
Even with coverage, its cost is controversial, with Sen. Bernie Sanders condemning the high price last month.
Sanders, who is chair of the Senate Health Committee, called the “outrageously high price” tag “unconscionable.”
“That would clearly not just be unaffordable to many seniors, it would be an absurd and unfair government policy,” wrote Sanders of the groundbreaking new drug.
What sets it apart from other treatments is that it targets the actual pathway of the disease — specifically, a protein called beta-amyloid, which is believed to be a cause of the progressive dementia that characterizes Alzheimer’s Disease.
It is only the second drug to do so; The first, Aduhelm, was approved in 2021,
In the Leqembi study, patients who received the new drug were found to “[have] a statistically significant reduction in brain amyloid plaque” as compared to the placebo group.
Those who took the new medication — which is administered over an hour-long intravenous infusion once every two weeks — saw a 27% slowing in the progression of the illness.
“Alzheimer’s disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones,” said Billy Dunn, M.D., director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research.
“This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease.”
However, Leqembi isn’t without its risks: “The odds for brain swelling and hemorrhage are far higher than any actual improvement,” Dr. Alberto Espay, a neurologist at the University of Cincinnati College of Medicine, told NBC News.
Espay had launched a petition in June calling for the Alzheimer’s treatment to not get full approval.
Related: Maureen McGovern on Living with Alzheimer's Disease: 'You Go One Day at a Time'
According to the FDA, risks include “temporary swelling in areas of the brain that usually resolves over time and may be accompanied by small spots of bleeding in or on the surface of the brain, though some people may have symptoms such as headache, confusion, dizziness, vision changes, nausea and seizure.”
And while the drug was shown to slow the progression of the disease in “patients with mild cognitive impairment or mild dementia stage of disease,” it was not tested on patients who are further along with dementia.
Never miss a story — sign up for PEOPLE's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from juicy celebrity news to compelling human interest stories.
For more People news, make sure to sign up for our newsletter!
Read the original article on People.

