New Alzheimer's Drug Reduces Cognitive Decline but May Cause Severe Side Effects, Study Finds
A new drug may slow the progression of Alzheimer's Disease, but it could come at a cost.
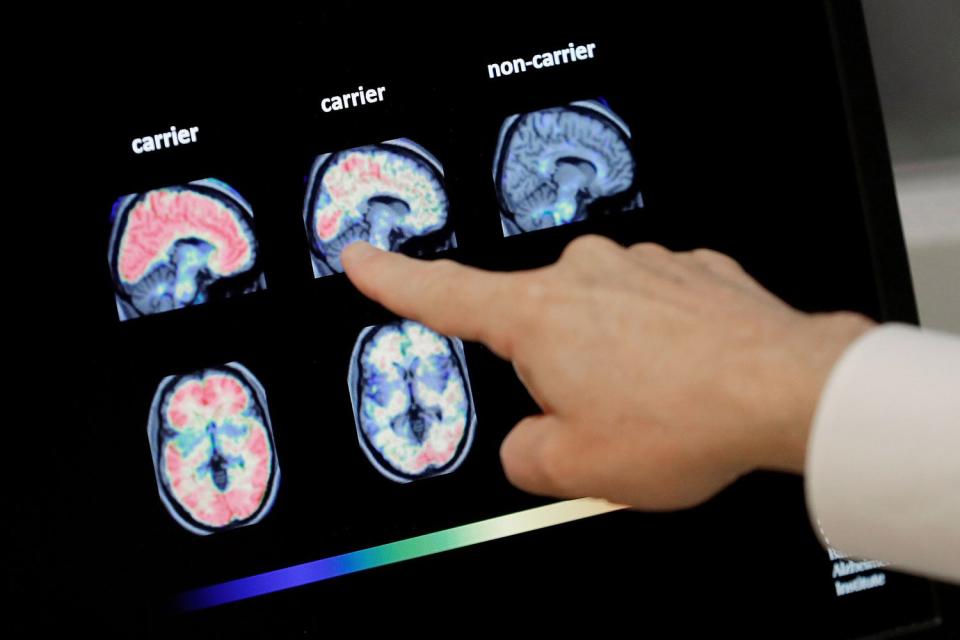
According to research published by the New England Journal of Medicine on Tuesday, the drug lecanemab slows cognitive decline.
"Lecanemab reduced markers of amyloid in early Alzheimer's disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events," the study's findings state.
But the publication said that "longer trials are warranted to determine the efficacy and safety" of the drug.
The results of the clinical trial come roughly two months after manufacturers of the drug, Eisai and Biogen, touted promising early results, according to The New York Times.
RELATED VIDEO: Maureen McGovern Reveals Symptoms of Alzheimer's Disease: 'My Inner Life Has Not Changed'
RELATED: FDA Approves New Alzheimer's Drug Despite Controversy Surrounding Its Effectiveness
In September, the pharmaceutical companies reported that in its trial of nearly 1,800 participants, cognitive decline among a group of volunteers who received the drug was reduced by 27%.
"The benefit is real; so too are the risks," Dr. Jason Karlawish, a co-director of the University of Pennsylvania's Penn Memory Center not involved in the research, told the Times.
Matt York/AP/Shutterstock
The drug is a monoclonal antibody that targets a protein, amyloid, which forms into plaques in the brains of those with Alzheimer's, but it can cause adverse effects including brain swelling and brain bleeding, according to CNN. Lecanemab is taken every two weeks by intravenous infusion, the Times reported.
"Lecanemab was generally well-tolerated," Dr. Marwan Sabbagh, an author of the study and professor at the Barrow Neurological Institute, said during Tuesday's news conference. "Most adverse events were infusion-related reactions, ARIA-H and ARIA-E and headache."
RELATED: Alzheimer's Patients and Their Caregivers Find Solace and Socialization in 'Memory Cafes'
Sabbagh added that such issues resolved within months.
The results of lecanemab's trial come months after the Food and Drug Administration approved another drug for Alzheimer's treatment.
Aduhelm was the first drug the agency had approved in 20 years for the disease, but its release was marred by controversy in the wake of studies that also showed it carried significant risks, per the Times.

